
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
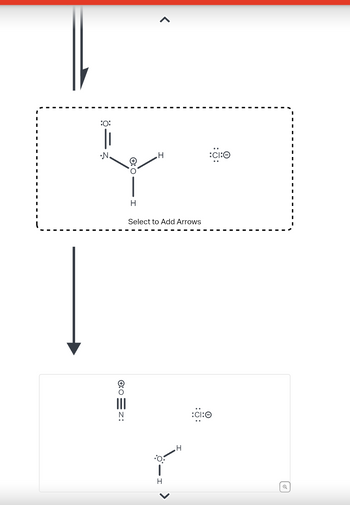
Transcribed Image Text::O:
||
H
4
'N•
Ⓒ:O z:
||||
:O I
<
H
Select to Add Arrows
|
H
>
H
อะเว:
:CI:0
Q
I
I
I
I
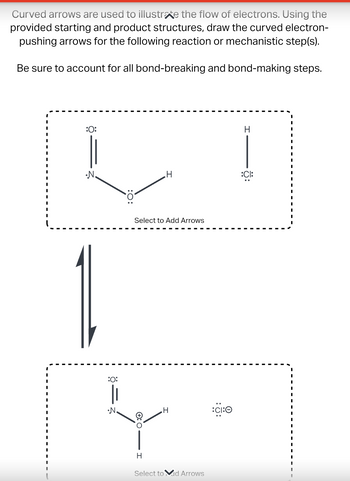
Transcribed Image Text:Curved arrows are used to illustrate the flow of electrons. Using the
provided starting and product structures, draw the curved electron-
pushing arrows for the following reaction or mechanistic step(s).
Be sure to account for all bond-breaking and bond-making steps.
:O:
•N.
:O:
•N.
:O:
Select to Add Arrows
Ⓒ:O-
H
H
H
Select to Mid Arrows
CI:O
H
:CI:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Use the following made up equivalencies to answer the following questions: • 30.5q equals 1s • 13.2s equals 1t • 82.1t equals 1p How many p are in 12654q?arrow_forwardWhat is the percent by weight (w/w%) of sugar in soda? Assume the average mass of sugar in soda is 22.0 g and the total mass is 370.0 g.arrow_forwardWhich two equations contain Boltzmann's constant? Select one or more: D a. b. C. d. E =k = k1/20 9=k9 r Ad = (2k + 1) (2k +1) 3 U = = NAKT 2 2arrow_forward
- fonj.acid అ,రవల Formula ha tormula HS 1.8.10.* HSO 0.012 HAO, 4.5164 Complete the table below. Bc Sure cach O7 Your ainouer entries has the Correct numbcr oA Signiticant digits. You may assume the tmparatore is 25°%.arrow_forwardheat (3 pts car of .COOH Na tBuOH/NH3arrow_forward2. Assume the density of air at room temperature is 1.2 g/mL. A student is holding two balloons filled with gases; one balloon filled with hydrogen gas (density = 0.082 g/mL) M and second balloon filled with carbon dioxide gas (density %3D 1.9 g/mL). Predict what %3D would happen if the student release both the balloons? (MO) onsritamonoidoib lo vienob orh onimstob of sisb gnivollol ors elsollo nabu A 2sbuloni bn how Tuov wod2old leoniin s ola 0 biopil Wod2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY