
Elements Of Electromagnetics
7th Edition
ISBN: 9780190698614
Author: Sadiku, Matthew N. O.
Publisher: Oxford University Press
expand_more
expand_more
format_list_bulleted
Question

Transcribed Image Text:4.37. The container in Fig. 4.19 is initially evacuated. Then the valve is opened, and
atmospheric air flows in, compressing the spring. The entire inflow and compres-
sion process is adiabatic. The temperature of the atmospheric air is 20°C. When
the process is over, the volume of the contained air is 10 m³, its pressure is 1 atm,
and the work done on the piston and spring system is 13 kJ. The air in the
container is perfectly mixed. What is the temperature of the air in the container?
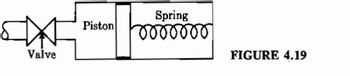
Transcribed Image Text:Valve
Spring
Piston ooooooo
FIGURE 4.19
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
hi, where did u get this R value from. these are the values i have
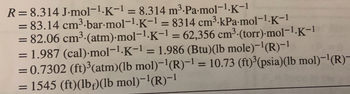
Transcribed Image Text:The image provides various units and equivalent values of the universal gas constant \( R \). Here are the expressions shown:
- \( R = 8.314 \, \text{J} \cdot \text{mol}^{-1} \cdot \text{K}^{-1} = 8.314 \, \text{m}^3 \cdot \text{Pa} \cdot \text{mol}^{-1} \cdot \text{K}^{-1} \)
- \( = 83.14 \, \text{cm}^3 \cdot \text{bar} \cdot \text{mol}^{-1} \cdot \text{K}^{-1} = 8314 \, \text{cm}^3 \cdot \text{kPa} \cdot \text{mol}^{-1} \cdot \text{K}^{-1} \)
- \( = 82.06 \, \text{cm}^3 \cdot (\text{atm}) \cdot \text{mol}^{-1} \cdot \text{K}^{-1} = 62,356 \, \text{cm}^3 \cdot (\text{torr}) \cdot \text{mol}^{-1} \cdot \text{K}^{-1} \)
- \( = 1.987 \, (\text{cal}) \cdot \text{mol}^{-1} \cdot \text{K}^{-1} = 1.986 \, (\text{Btu}) (\text{lb mole})^{-1} (\text{R})^{-1} \)
- \( = 0.7302 \, (\text{ft})^3 (\text{atm}) (\text{lb mol})^{-1} (\text{R})^{-1} = 10.73 \, (\text{ft})^3 (\text{psia}) (\text{lb mol})^{-1} (\text{R})^{-1} \)
- \( = 1545 \, (\text{ft}) (\text{lb}_f) (\text{lb mol})^{-1} (\text{R})^{-1} \)
These are different units in which the gas constant can be expressed, used depending on the system of units (SI, imperial,
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
hi, where did u get this R value from. these are the values i have
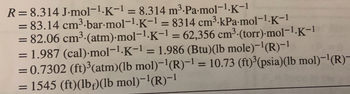
Transcribed Image Text:The image provides various units and equivalent values of the universal gas constant \( R \). Here are the expressions shown:
- \( R = 8.314 \, \text{J} \cdot \text{mol}^{-1} \cdot \text{K}^{-1} = 8.314 \, \text{m}^3 \cdot \text{Pa} \cdot \text{mol}^{-1} \cdot \text{K}^{-1} \)
- \( = 83.14 \, \text{cm}^3 \cdot \text{bar} \cdot \text{mol}^{-1} \cdot \text{K}^{-1} = 8314 \, \text{cm}^3 \cdot \text{kPa} \cdot \text{mol}^{-1} \cdot \text{K}^{-1} \)
- \( = 82.06 \, \text{cm}^3 \cdot (\text{atm}) \cdot \text{mol}^{-1} \cdot \text{K}^{-1} = 62,356 \, \text{cm}^3 \cdot (\text{torr}) \cdot \text{mol}^{-1} \cdot \text{K}^{-1} \)
- \( = 1.987 \, (\text{cal}) \cdot \text{mol}^{-1} \cdot \text{K}^{-1} = 1.986 \, (\text{Btu}) (\text{lb mole})^{-1} (\text{R})^{-1} \)
- \( = 0.7302 \, (\text{ft})^3 (\text{atm}) (\text{lb mol})^{-1} (\text{R})^{-1} = 10.73 \, (\text{ft})^3 (\text{psia}) (\text{lb mol})^{-1} (\text{R})^{-1} \)
- \( = 1545 \, (\text{ft}) (\text{lb}_f) (\text{lb mol})^{-1} (\text{R})^{-1} \)
These are different units in which the gas constant can be expressed, used depending on the system of units (SI, imperial,
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Consider the heavily insulated barrel shown below. • What is the mass flow rate of water that exits the barrel at 3? • What phase(s) are present at the exit at 3? • What is the temperature at the exit at 3? • What is the diameter at the exit at 3?arrow_forward4.7. In an ideal liquefaction system the gas to be liquefied is first compressed isothermally and reversibly from its initial state to a high pressure, whereupon the gas is expanded through a reversible adiabatic expander to the saturated-liquid condition. To what absolute pressure must nitrogen gas be compressed at the end of the isothermal compression assuming that the nitrogen gas obeys the relation pV= ZNRT, where Z is the compressibility factor? The nitrogen gas is initially available at 0.101 MPa and 300 K.arrow_forwardA 653 g/h stream of methyl alcohol, also called methanol, at 6.0atm 10.0C was held at constant pressure, vaporized, and brought to 241.0C. At what rate must heat, be supplied to this system ? Assume that methyl alcohol vapor behaves ideally for the temperature range and pressure given.arrow_forward
- Superheated steam from boiler expands in turbine from initial pressure p₁ = 230 bar and initial temperature T₁ = 577 °C to pressure p₂ = 1 MPa. Isentropic efficiency of turbine ns is 0.8. Steam mass flow rate am,h is 146 kg/s. Read from h,s-chart Enthalpy of superheated steam h Enthalpy after isentropic expansion h₂,s Calculate: Enthalpy at the turbine outlet h₂ Power of turbine P MWarrow_forwardHelium is compressed through a compressor steadily. At the inlet the pressure is and the temperature is . At the exit the pressure is and the temperature is . The power input is and the heat loss rate is during this process. Neglect the kinetic and potential energy changes. Assume helium is ideal gas with a constant specific heat and its specific heat ratio , which means that enthalpy can be calculated using . Select the simplified the energy balance equation for this process_________ A. B. C. D.arrow_forwardA gas flows steadily through a rotary compressor at a temperature of 16 ⁰C, a pressure of 100kPa, and an enthalpy of 392.2 kJkg-1. The gas leaves the compressor at a temperature of 245⁰C, a pressure of 0.6 MPa and an enthalpy of 534.5 kJkg-1. There is no heat transfer to or fromthe gas as it flows through the compressor. Using the steady flow equation appropriate toeach case, evaluate the external work done per unit mass of gas: a) when the inlet and exit velocities of the gas are negligible b) when the inlet velocity of the gas is 80 ms-1 and the exit velocity of the gas is 160 ms-1.arrow_forwardRefrigerant R-134a to the compressor of a refrigeration machine It enters at 140 kPa pressure and -10 °C, and exits at 1 MPa pressure. Volumetric flow of the refrigerant entering the compressor It is 0.23 m³/minute. The refrigerant enters the throttling valve at a pressure of 0.95 MPa and at 30 °C, and exits the evaporator as saturated steam at -18 °C. Adiabatic efficiency of the compressor It is 78%. Show the cycle in the T-s diagram. And; a) Calculate the power required to run the compressor. b) Calculate the heat absorbed per unit time from the cooled medium. ( COPSM=? ) c) Calculate, between the evaporator and the compressor, how much the pressure of the refrigerant drops and how much the heat gain.arrow_forwardHelium is compressed through a compressor steadily. At the inlet the pressure is and the temperature is . At the exit the pressure is and the temperature is . The power input is and the heat loss rate is during this process. Neglect the kinetic and potential energy changes. Assume helium is ideal gas with a constant specific heat and its specific heat ratio , which means that enthalpy can be calculated using . Calculate the work per unit mass _________arrow_forwardSteam at a rate of 200 kg/min enters a turbine at 350°C and 40 bar through a 7.5-cm internal diameter pipe. The turbine operation is adiabatic, and the effluent leaves as saturated water at 5 bar through a 5-cm diameter pipe. 1. Calculate the work produced by the turbine in kW. 2. What is the enthalpyand phase of the effluent stream? 21 If it leaves the turbine at 75C and 5 bar 22 If it leaves the turbine at 30C and 5 bar 7.5arrow_forwardNo Handwriting pleasearrow_forwardAn insulated tank A of 0.6 m3 volume is connected to an insulated tank B of 0.3m3 volume. Initially, tank A contains steam at 0.2 MPa and 200 °C, and tank B contains wet steam at 0.5 MPa and 85% quality. The valve is opened and flow occurs until equilibrium (both mechanical and thermal) is attained. Estimate the final pressure.arrow_forwardA rigid tank of volume 10 m³ initially contains saturated water vapor at a temperature of 120 °C. Steam at a pressure 1.2 MPa and a temperature of 400 °C enters the tank through a valve in steam line that is connected to the tank until the final pressure in the tank is 800 kPa, at which time the temperature is 200 °C. All kinetic and potential energy effects can be neglected. A schematic of the problem and properties at all state points except state 1 are shown in the figure below. All of the properties at state 2 and the inlet state i are provided on the figure. Initial State in Tank T₁-120 °C, Sat. vapor u₁=? kJ/kg V₁=? m³/kg Pi=1.2 MPa, Ti-400 °C hi-3261.3 kJ/kg V=10 m³ Final State in Tank T: 200 °C, P₂-800 kPa u₂= 2631.1 kJ/kg v₂=0.26088 m³/kg Qout For Question 6: The initial specific internal energy, u1, of the saturated vapor in the tank in kJ/kg isarrow_forwardarrow_back_iosarrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY