
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
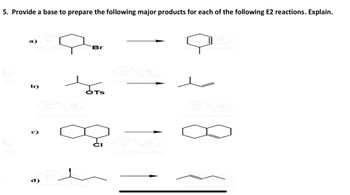
Transcribed Image Text:5. Provide a base to prepare the following major products for each of the following E2 reactions. Explain.
Chemistr S Br
b)
@
OT
“Chérsistry SE
Chemistry Skips
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A student found that heating any one of the isomers shown here resulted in scrambling of the deuterium to all three positions on the five-membered ring. Propose a mechanism to account for this observation.arrow_forwardDetermine whether the following reaction is likely to proceed by an E1 or E2 mechanism. CH;CH;OH Br E1 None E2arrow_forwardWhat is the best way to remeber the differences between Sn1, Sn2, E1 and E2 reations?arrow_forward
- 1) a) Propose a detailed mechanism for the reaction of 2-bromo-2,3-dimethylbutane with sodium methoxide (NaOCH3), showing formation of the major product. Please include: 1) a perspective diagram of the substrate 2) a Newman projection 3) a perspective diagram illustration of the transition state 4) curved arrows, lone pairs, formal/partial charges etc. as appropriate Special Request: Please don't do anything with the spectator ion. b) Draw a simple ('flat,' no dashes/wedges required) Lewis diagram of the minor product. 1) Why is it minor? 2) How would you alter the reaction conditions given to favor the minor product?arrow_forwardWill each of the following reactions follow an El or E2 mechanism? to to HO,arrow_forward1. Take a close look at the following reactions. (a) Draw the product in the box below. (b) Provide the mechanism in the space below the reaction scheme. H₂O, H₂SO4arrow_forward
- Which of the following substrates can undergo an E2 step with H2N¯ as the base? For those that can, draw the curved arrow notation and the products. (a) (b) CI (c) (d) (e) `Br Brarrow_forwardEach of the four reactions below undergoes an E2 mechanism. How many possible alkene products can be produced in each reaction? Hint: think about all major and minor possible products. O Hold and drag to reorder A) = 1 B) = 3 C) = 3 D) = 2 CI tBuOK A) CI NaOEt B) NaOEt C) CI CI NaOEt D) Pharrow_forwardIndicate whether the reaction below is an oxidation, reduction, or neither.arrow_forward
- 3. Which of the following has the ability to react via both SN1 and SN2 mechanism? Br A) B) Br C) D) yo Br CH3BRarrow_forward5) Predict the major product formed in the following multistep reaction sequence. CI 1) NaNH2 2) NaNO2, HCI F3C CI 3) CuBrarrow_forwardThe reaction of (S)-2-bromobutane with sodium hydroxide can occur via either an SN1 or SN2 mechanism. Draw the arrow pushing for each of these mechanisms.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY