
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Give me a clear handwritten Detailed Solution
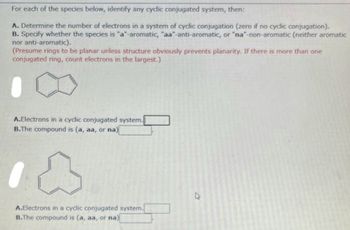
Transcribed Image Text:For each of the species below, identify any cyclic conjugated system, then:
A. Determine the number of electrons in a system of cyclic conjugation (zero if no cyclic conjugation).
B. Specify whether the species is "a"-aromatic, "aa-anti-aromatic, or "na"-non-aromatic (neither aromatic
nor anti-aromatic).
(Presume rings to be planar unless structure obviously prevents planarity. If there is more than one
conjugated ring, count electrons in the largest.)
A.Electrons in a cyclic conjugated system.
B.The compound is (a, aa, or na)
A.Electrons in a cyclic conjugated system.
B.The compound is (a, aa, or na)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- kindly provide complete and full solution. i won't like your solution if it is incomplete or not clear enough to read.arrow_forwardA student determined the concertration of NaOCI in a commercial bleach sample using the same technique described in section "F" of the Halogens and Their Compounds lab. The student required 18 drops of 0.01M Na25203 to reach the end point. What is the concentration (reported in percentage) of NaOCI in the bleach sample?arrow_forwardA 0.8250 g of soda ash sample was dissolved in distilled H₂O and diluted to 250-mL using a volumetric flask. This solution was labeled as dilute soda ash solution (DSAS). a. A 50.00 mL aliquot portion of the dilute soda ash solution needed 5.20 mL of 0.1200 M HCI to reach the phenolphthalein end point. About 2-3 drops of methyl orange to the same mixture and it needed 10.00 mL of 0.1200 M HCI to reach the methyl orange end point. 1. Calculate the number of millimoles of HCI needed to reach the phenolphthalein end point. 2. Calculate the number of millimoles of HCI needed to reach the methyl orange end point. 3. What is the %Na₂CO, and %NaHCO, present in the soda ash sample? b. From the dilute soda ash solution (DSAS) two 50-mL portions were obtained. The first portion needed 5.20 mL of 0.1200 M HCI to reach the phenolphthalein end point. The second 50-ml portion needed 15.20 mL to reach the methyl orange end point. 4. Calculate the number of millimoles of HCI needed to reach the…arrow_forward
- physical chemistry, please solve question 2arrow_forwardgrammarly-G X * Content oard.odu.edu/ultra/courses/_393452_1/cl/outline F2 Question Completion Status: X GA student wan X 1 QUESTION 3 A student wants to determine the solubility of water and copper (1) bromide (a green solid) in ethyl acetate (a colorless organic liquid). She places 1 mL of ethyl acetate into each of two test tubes. To one, she adds 5 drops of water to it. To the other, she adds a small scoop of copper(1) bromide. 8.0 F3 2 - Which test tube represents the ethyl acetate and A. Test tube 1 copper(1) bromide if the copper(1) bromide is insoluble in ethyl B. Test tube 2 acetate? C. Test tube 3 D. Test tube 4 - Which test tube represents the mixture of the ethyl acetate and water if the water is insoluble in ethyl acetate? $ Question Com X C Chegg Search x 3 Which test tube represents the mixture of the ethyl acetate and water if the water is soluble in ethyl acetate? - Which test tube represents the ethyl acetate and copper(1) bromide if the copper(1) bromide is soluble…arrow_forwardQ4/ Prepare 100ml of 0.1 a/from NaC stock solution (5M). How many milliliters which required from stock solution?.arrow_forward
- Question 2 (b) Name and discuss five stages in which gravimetric analysis procedure may be dividedarrow_forwardDeterminati an 25 ml by dissolving 110n (111) per 1 This (as ml 750ml experiment Solution The to of was The a and process Q on added The portion layer taken 1 volumetric diluted Litre Colution Two These of in diluted ml soo 1% ETA of Question A. reaction an Solution 0₁ 446 9 of A. R. hydrated sulphate, (NH4) Fe (504) ₂ · 12H₂0 2 of distilled then of wertel) 0,0446 g/L of lion Absorbance was 250 ml in to ·layers frask was Is 2. 3. Was Chlore form and were 5 ml, 10 ml, 15 ml por tions 4. Iron • iron (111) four oxine it mixture the done. (111) seperatory conducted Volume SMT as 10 ml 15 ml 20 ml Plo + absorbance formed readings a (A.R) Jayer measured the por tions Solution was Water diluted out 20 ml were then Each of these mark against funnel as was after with for 8- ity droxy quinolate. follows. to iron (111) solution was prepared of then Calibration was ) 10 obtain collected. ammonium F. W-481,979 then these Absorbance 0,076 0,169 9185 0₁234. Shook. the shaking added portions…arrow_forwardThe Kjeldahl method was used to determine the nitrogen content of a protein. A 275 µL aliquot of a 47.0 mg/mL protein solution was digested in boiling sulfuric acid. The solution was made basic and the liberated NH, was collected in 5.00 mL of 0.0512 M HCl. A volume of 7.00 mL of 0.0174 M NaOH was required to react with the excess HCl. Calculate the weight percent of nitrogen in the protein. weight percent: wt% Narrow_forward
- The equivalence point is defined as moles acid = moles base. So first, let's calculate the initial moles of base we used. molpase Mpase = Volsoiution Molarity base = 0.100 (M) Volume of solution= _50.00 mL Moles of base = _5.00 mmol So, we need 5.00 mmol of acid (titrant) to be delivered to the analyte. Since we know the molarity of the acid and moles of acid, we just need to solve for the volume of acid (titrant) to be added. molacid Voladded Macid Molarity acid = . 0.200 (M) Moles of acid = _5.00 (mmol) Volume of acid needed to reach equivalence point (mL)arrow_forwardWhat is the easiest process to get to the result?arrow_forwardb) A solution was prepared by dissolving 1.5 g of Acetic acid (CH,COOH) in a 250cm solution. 50cm of this solution was allowed to react with 25cm of 0.1 NaOH solution (C-12, H-1, O-16) i) Determine the concentration of the acetic acid solution in moldm ii) iii) Write down an equation for the reaction. Calculate the number of moles of CH,COOH left unreacted and the number of moles of CH,COONA formed. iv) What name is given to the solution formed when all the NaOH has reacted? Calculate the Pp" of the solution in (iv) above (ka for CH,COOH = 1.8 x 10)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY