
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question

Transcribed Image Text:Q1: Find the molar conductivity (in S.cm².mol) of a 0.00100 M solution of KCI if its
conductivity is 1.40×10-3 S.cm-¹ at 298 K.
a) 0.0140
b) 140.
a) 0.0207
c) 1.40
Q2: The molar conductivity of a 0.0213 M solution of a weak base (BOH) is 83.4 S.cm².mol-¹.
What is the value of the degree of dissociation if the sum of the ionic conductivities at infinite
dilution for B and OH is 237 S.cm².mol-¹?
b) 0.484
a) 0.404
c) 0.352
d) 1400.
c) 1.00
d) 1.36x10-5
Q3: If the transport number (t+) for an electrolyte is 0.55. The ratio of the ionic mobility of the
cation to the anion will be:
b) 1.78
c) 0.934
e) 14.0
d) 1.22
physical
chemistry
e) 2.07x10-5
Q4: Find the mean activity coefficient (y+) for Na2SO4. The activity coefficients for Na* and
SO4 are 0.913 and 0.695, respectively.
a) 0.634
b) 0.534
d) 0.734
e) 0.941
e) 0.834
Expert Solution
arrow_forward
Step 1
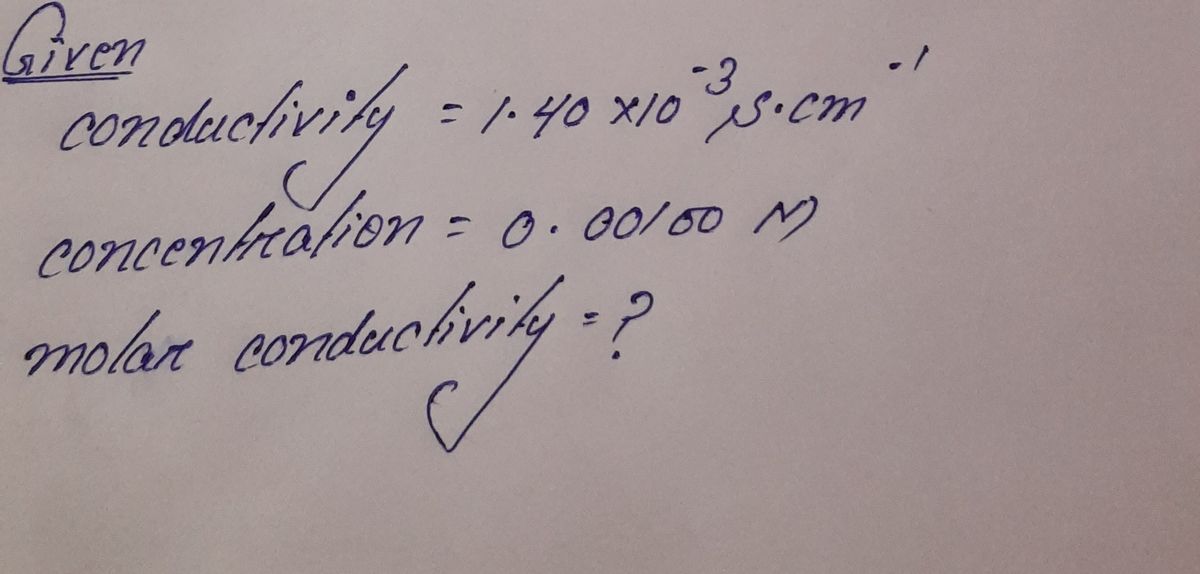
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A scientist has two containers. Inside each container is a blue liquid. The liquids are substances. What can the scientist do to help find out whether these two liquids are both the same substance?arrow_forwardFact, Law, Hypothesis, or Theory? __ An object less dense than the air flies up on its own. __ Atoms make and break chemical bonds by transferring or sharing their valence electrons to satisfy the octet rule. __ A firework is seen to burn with a green flame.arrow_forwardThe history of science is important to current and future research. O True O Falsearrow_forward
- Choose the homogeneous mixture from the list below. O soda (pop) air concrete trail mix bloodarrow_forwardCHEMISTRY Calculating Density Density is the compactness of matter. Density describes the quantity of matter (mass) per unit of volume (3-dimensional space). Density is calculated as the mass of the matter divided by volume. The symbol for density is Greek letter rho (p). The units for mass are in grams (g) and the units for volume is cubic centimeter (cm³). It should be noted that 1 cm³ is equal to 1 ml of volume. 1 cm³= 1 ml. The units for density are g/cm³. Part 1 Determine the density of the new liquid materials created in the Secret Density Research Laboratory. Type the value (no units) of the density into the boxes in the density column. Report density to the 2nd number after the decimal. Use a zero to the lef of the decimal for values less than 1.00. Examples: 2.35, 0.75, 1.90 Material name Harrisonite Waltonium Kellese Wheelernone Osborneum Popebrillium Mass (g) 430 590 260 m P=V 620 380 510 Volume (cm³) 537 454 236 364 633 340 Density (g/cm³) SAMSUNGarrow_forwardHey ! Please help me with all its all very short type questions so do it all on a plain white paper please I'll Upvote you surely.thankyouarrow_forward
- description of an element at the particulate levelarrow_forward2nd - Which of the following is false for mixtures? a) Mixtures have the properties of the substances that make them up. B) Sandy water is an example of a heterogeneous mixture. NS) Cologne is an example of a homogeneous mixture. D) Mixtures are separated into their components by chemical method. TO) Mixtures are divided into homogeneous and heterogeneous.arrow_forwardWhat are basic concepts in chemistry? Give 5 examples and explain each.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY