
Interpretation:
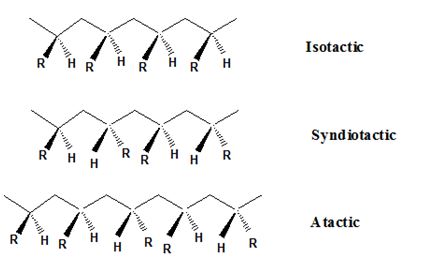
Predict the result of the catalytic hydrogention of natural rubber and will the product obtained show syndiotactic, atactic or isotactic behaviour.
Concept introduction:
Isotactic, syndiotactic, and atactic are the stereochemical forms. The
Syndiotactic are the macromolecules in which the (-R) groups are arranged in an alternate manner along the long chain of the polymer. Gutta-percha is also an example for Syndiotactic polymer.
In atactic form, the substituents are placed in a random manner along the long chain.
The important point to note here is that the polymer obtained from the chain-growth
The catalytic hydrogenation natural rubber requires the breaking of double bond and the addition of hydrogen. The double bond of
Therefore, it breaks and hydrogen adds to it. When the hydrogen adds, then the double bonds are replaced with the single bonds. The hydrogenation of double bond releases energy therefore, known as an exothermic reaction. The heat released is called the heat of hydrogenation.
The catalyst used for the purpose of hydrogenation can be Ra-Ni (Raney-Nickel), PtO2 (Platinum oxide), Pd-C (Palladium on carbon) etc. They can be used to enhance the
Trending nowThis is a popular solution!

Chapter 31 Solutions
Organic Chemistry
- Write the steps for formation of tetrachloromethane (CCl4) from the reaction of methane with Cl2 + hn.arrow_forwardTeflon can be made from tetrafluoroethylene by means of a free radical polymerization. In the reaction, termination occurs only by combination and not by disproportionation. Explain this observation.arrow_forwardQ18. i)Write the general formulae of the Alkene and give three examples with name and Structural formulae. ii) A mass of 0.750 grams of Iron metal is produced from Fe2 solution in 50.0 minutes. Compute the electric current supplied to the cell during the electrolysis. [F= 96500 C mol]arrow_forward
- How many kg of methane (CH4) are produced from 25.0 kg of hydrogen in the following catalytic methanation? 3 H2 + CO - CH4 + H2O 1) 115 kg 2) 74.3 kg 3) 134 kg 4) 25.0 kg 5) 66.3 kgarrow_forwardConsider the hydrotreating of this organic substance:H2NCH2CSCH2COOH +___ H2(g) → (a) Complete a balanced chemical equation to producethe most common hydrogen compounds of N, S, andO and a saturated hydrocarbon.(b) Why is more hydrotreating needed after coking thanafter hydrocracking?arrow_forwardGive the structure of the condensation copolymer made from the following monomers: HOOCCH2CH2COOH and H2NCH2CH2CH2CH2CH2CH2NH2arrow_forward
- 85. Propane, C3H8, is a hydrocarbon that is commonly used as a fuel. (a) Write a balanced equation for the complete combustion of propane gas. (b) Calculate the volume of air at 25 °C and 1.00 atmosphere that is needed to completely combust 25.0 grams of propane. Assume that air is 21.0 percent O2 by volume. (Hint: We will see how to do this calculation in a later chapter on gases—for now use the information that 1.00 L of air at 25 °C and 1.00 atm contains 0.275 g of O2 per liter.) (c) The heat of combustion of propane is −2,219.2 kJ/mol. Calculate the heat of formation, ΔH∘fΔHf° of propane given that ΔH∘fΔHf° of H2O(l) = −285.8 kJ/mol and ΔH∘fΔHf° of CO2(g) = −393.5 kJ/mol. (d) Assuming that all of the heat released in burning 25.0 grams of propane is transferred to 4.00 kilograms of water, calculate the increase in temperature of the water.arrow_forwardH-bonding is present in carbohydrates and proteins. Given the following structures: (a) glucose, a monomer of carbohydrates, and (b) glycine, an amino acid that is a protein building block, highlight the sites for H-bonding. H-C-OH HO-C-H Н-с-ОН H-C-OH N-C CH2OH 0-H a. Glucose b. Glycine HICI H.arrow_forwardA sample petrol gave 85% of carbon and 15% of hydrogen gas. Calculate the weigth of air required for complete combustion of 1kg of petrol.arrow_forward
- The name carbohydrate comes from the fact that many simple sugars have chemical formulae that look like water has simply been added to carbon. (The suffix hydrate from the Greek word hydor ("water") means "compound formed by the addition of water.") The actual chemical structure of carbohydrates doesn't look anything like water molecules bonded to carbon atoms (see sketch at right). But it is nevertheless possible to chemically extract all the hydrogen and oxygen from many simple carbohydrates as water, leaving only carbon behind. If you search the Internet for "reaction of sulfuric acid and sugar" you will find some impressive videos of this. Suppose you had 300.g of ordinary table sugar, which chemists call sucrose, and which has the chemical formula C12H22O11 . Calculate the maximum mass of water you could theoretically extract. Be sure your answer has a unit symbol, and round it to the correct number of significant digits.arrow_forwardThe name carbohydrate comes from the fact that many simple sugars have chemical formulae that look like water has simply been added to carbon. (The suffix hydrate from the Greek word hydor ("water") means "compound formed by the addition of water.") The actual chemical structure of carbohydrates doesn't look anything like water molecules bonded to carbon atoms (see sketch at right). But it is nevertheless possible to chemically extract all the hydrogen and oxygen from many simple carbohydrates as water, leaving only carbon behind. If you search the Internet for "reaction of sulfuric acid and sugar" you will find some impressive videos of this. Suppose you had (200. g) of ordinary table sugar, which chemists call sucrose, and which has the chemical formula C12H₂2011. Calculate the maximum mass of water you could theoretically extract. Be sure your answer has alunit symbol, and round it to 3 significant digits. 0 HO CH₂OH OH OH OH The actual chemical structure of glucose.arrow_forward21.) Calculate the enthalpy of hydrogenation of benzene to cyclohexane from the following reactions A,H (kJ/mol) C6H6 (1) + 15/2 02 (g) → 6 CO2 (g) + 3 H20 (1) C6H12 (1) + 9 02 (g) → 6 CO2 (g) + 6 H20 (1) H2 (g) + ½ 02 (g) → H2O (1) -3268 -3920 -285.83 a.) -205 kJ/mol b.) -1507 kJ/mol c.) -938 kJ/mol d.) -366 kJ/molarrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning


