
Tutorials in Introductory Physics
1st Edition
ISBN: 9780130970695
Author: Peter S. Shaffer, Lillian C. McDermott
Publisher: Addison Wesley
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 27.2, Problem 1aTH
To determine
To Describe: Whether, an ideal gas process satisfies the given conditions or not.
Expert Solution & Answer
Explanation of Solution
Introduction:
The process with constant temperature and with some
Take an example of piston cylinder arrangement with an ideal gas. Gas is allowed to expand and contract. The heat is supplied or extract from the cylinder . This expands or contracts the piston slowly and produce work without change in temperature.
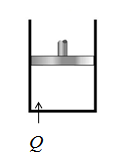
Conclusion:
Yes, in isothermal process the temperature is constant and heat is transferred.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
First Law of Thermodynamics
PROCEDURES: in the pictures attached
A.When gas expands, Is the (W) work done by the system or (W) work done on the system? Explain your answer.
B.Compare the temperature outside (surroundings) and inside (system) the bottle before pouring the boiling water into the bucket.
C.Compare the temperature outside (surroundings) and inside (system) the bottle after pouring the boiling water into the bucket.
Answer the following question comprehensively. Show your complete solution.
a. Suppose a woman does 500 J of work and 9000 J of heat transfer occurs into the environment in the process. What is the decrease in her internal energy, assuming no consumption of food?
An ideal gas undergoes two thermodynamic processes as shown in
FIGURE 1.
a. Name the processes AB and BC.
b. If the initial temperature is 40°C, find the temperatures at B and C.
c. Find the total work done
d. What is the total change in internal energy for these processes
Chapter 27 Solutions
Tutorials in Introductory Physics
Ch. 27.1 - Prob. 1aTHCh. 27.1 - In this process, which of the quantities P, V, n,...Ch. 27.1 - Consider the following incorrect student...Ch. 27.1 - Explain why it is not possible to use the ideal...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - Prob. 2cTHCh. 27.2 - Prob. 1aTHCh. 27.2 - Prob. 1bTHCh. 27.2 - Prob. 1cTH
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Suppose a monatomic ideal gas is changed from state A to state D by one of the processes shown on the PV diagram. 1. The gas follows the constant-temperature path AC followed by the constant-pressure path CD. What is the total work done on the gas ? What is the total change in internal energy of the gas during the entire process? What is the total heat flow into the gas?arrow_forwardtemperatures, including absolute zero. Show how you draw the graphs with formulas and explain when necessary. b) In the adiabatic process for Fermi or Bose gas: VT2 Sbt., PV- Sht. and T/P Sbt. and photon gas PV-Sbt., VT' Sbt. and T/P- Sbt. Prove these formula.arrow_forwardBoyles Law states that when a sample of gas is compressed at a constant temperature, the product of the pressure and the volume remains constant: PV=C. A. Find the rate of change of volume with respect to pressure. B. A sample of gas in a container at low pressure and is steadily compressed at constant temperature for 10 minutes. Is the volume decreasing more rapidly at the beginning or the end of the 10minutes? Please Explain.. C. Prove that the isothermal compressibility is given by B=1/P.arrow_forward
- Give an equation for the infinitesimal change in entropy, ??, of a system in terms of the heat transfer ??? and the temperature ? at which the heat transfer occurs. Explain the sign convention.ii. A litre of water at 20 °C is placed in a fridge at 5 °C. Calculate the change in entropy of the water, including the sign, once all the water has come into thermal equilibrium.iii. Calculate the change in entropy of the fridge from part ii, including the sign.iv. Demonstrate that the Second Law has been obeyed in the process described in ii and iii.arrow_forwardpin 6. A long pin is now used to hold the piston in place as shown in the diagram. The cylinder is then placed into boiling water. The valve remains closed for the whole time. a. Does the temperature of the gas increase, decrease, or remain the same? b. Sketch this process in the PV diagram. Using arrow, indicate the direction of the process on your graph. c. Explain why for this particular situation, it is impossible to determine the pressure of the gas as you did on question 1 (that is, by considering the free-body-diagram of the piston).arrow_forwarda- A box of gas has 17 distinguishable particles and 168 cells. Calculate the number of microstates. State your answer in exponential form. b. Choose the correct statements that are related to the video example of a box with a movable partition. a. Maximizing Ωtotal determines the macrostate with the maximum number of microstates. b. Maximizing Ωtotal determines the macrostate with the highest probability. c. Maximizing Ωtotal determines the macrostate that is the equilibrium state. d. Maximizing Ωtotal determines the macrostate which maximizes the number of microstates in the left half of the box.arrow_forward
- Answer the following statements/questions comprehensively. Show your complete solution. a. Find the efficiency of a heat engine that absorbs 2500 J of energy from a hot reservoir and exhausts 1000 J to a cold reservoir during a cycle. b. Suppose a woman does 500 J of work and 9000 J of heat transfer occurs into the environment in the process. What is the decrease in her internal energy, assuming no consumption of food?arrow_forwardDirections: Illustrate using arrows the given situation below. Use a separate paper for your answer. 1. A gas, while expanding, absorbs 25 J of heat and does 243 J of work. The change in the internal energy of the gas is - 218 J. 2. In compressing a gas, 355 J of work is done on the system. At the same time,185 J of heat escapes from the system.arrow_forwardPart B A container holds a sample of ideal gas in thermal equilibrium, as shown in the figure. (Figure 1) One end of the container is sealed with a piston whose head is perfectly free to move, unless it is locked in place. The walls of the container readily allow the transfer of energy via heat, unless the piston is insulated from its surroundings. Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas after the following process? "Immerse the container into a large water bath at the same temperature, and very slowly push the piston head further into the container." • View Available Hint(s) O point 1 O point 2 O point 3 O point 4 O point 5 O point 6 O point 7 O point 8 Submit Previous Answers X Incorrect; Try Again; 4 attempts remaining The volume of the gas cannot remain constant because the piston head has moved further into the container. Part C Starting from equilibrium at point 0, what point on the pV diagram will describe the ideal gas…arrow_forward
- 13 moles of monatomic ideal gas undergoes a thermodynamic process from point a topoint c, as shown by the PV diagram in Figure 1. i) Determine the work done ON the gas from point a to point c ii) Find the temperature of the gas at point barrow_forwardAnswer the questions that follow. 1. Explain how the Zeroth law of thermodynamics in the figure A and B. 2. Metal lids on glass jars can often be loosened by running hot water over them. How is this possible? 3. Does it really help to run hot water over a tight metal lid on a glass jar before trying to open it? Explain your answer. 4. When a cold alcohol thermometer is placed in a hot liquid, the column of alcohol goes down slightly before going up. Explain why.arrow_forwardAnswer the following questions. Show your complete solution if necessary. 1. Jogging every day is good for your health. Assume that when you jog a work of 500 kJ is done and 230 kJ of heat is given off. What is the change in the internal energy of your body? 2. What is the change in the internal energy of a system when a total of 150.00 J is transferred by heat from the system and 159.00 J is done by work on the system?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON