
Concept explainers
(a)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
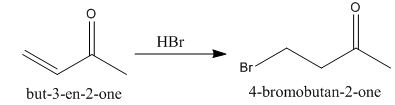
Explanation of Solution
The principal organic product obtained when

Figure 1
In this reaction, the addition of
The principal organic product obtained when
(b)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
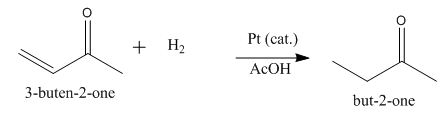
Explanation of Solution
The principal organic product obtained when

Figure 2
In this reaction, the addition of
The principal organic product obtained when
(c)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
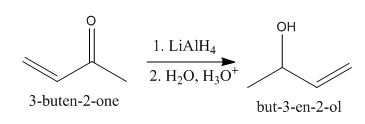
Explanation of Solution
The principal organic product obtained when

Figure 3
In this reaction, the addition of
The principal organic product obtained when
(d)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
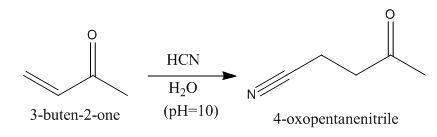
Explanation of Solution
The principal organic product obtained when

Figure 4
In this reaction, the addition of
In the product, hydrogen will add to that carbon of the double bond that has the least number of hydrogens.
The principal organic product obtained when
(e)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
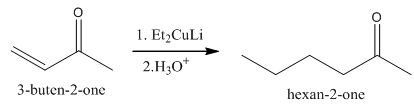
Explanation of Solution
The principal organic product obtained when

Figure 5
The reaction of
The principal organic product obtained when
(f)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
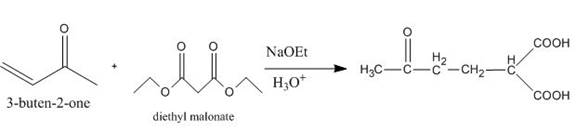
Explanation of Solution
The principal organic product obtained when

Figure 6
The reaction of
The principal organic product obtained when
(g)
Interpretation:
The principal organic product expected when
Concept introduction:
The
Answer to Problem 22.55AP
The principal organic product obtained when
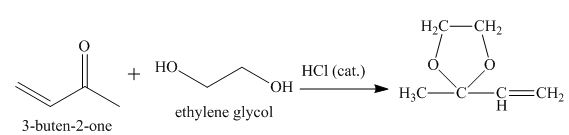
Explanation of Solution
The principal organic product obtained when

Figure 7
The reaction of
The principal organic product obtained when
(h)
Interpretation:
The principal organic product expected when
Concept introduction:
Diels-Alder reaction is a cycloaddition reaction. The reaction is known as a
Answer to Problem 22.55AP
The principal organic product obtained when
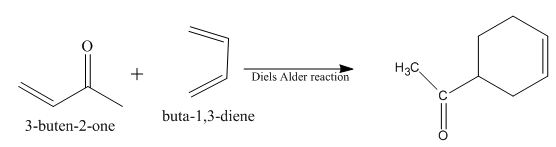
Explanation of Solution
The principal organic product obtained when

Figure 8
The reaction of
The principal organic product obtained when
Want to see more full solutions like this?
Chapter 22 Solutions
Organic Chemistry
- Predict the products of the following acid-base reactions. If the equilibrium would not result in the formation of appreciable amounts of products, you should so indicate. In each case label the stronger acid, the stronger base, the weaker acid, and the weaker base: (a) CH3CH=CH2 + NANH2 (d) CH3C=C: + CH;CH2OH → (e) CH3C=C:- + NH¾CI – | (b) CH;C=CH + NaNH2 (c) CH3CH2CH3 + NANH2 → | HASarrow_forwardPredict the major products (including stereochemistry) when cis-3-methylcyclohexanol reacts with the following reagents. (a) concentrated HBr (b) TsCl/pyridine, then NaBrarrow_forward(a) (b) HO N N-H Catalytic H+ (-H₂O) ? (c) NH2 HO N (d)arrow_forward
- (a) A hydrocarbon isolated from fish oil and from plankton was identified as 2,6,10,14-tetramethyl-2-pentadecene. Write its structure.(b) Alkyl isothiocyanates are compounds of the type RN C S. Write a structural formula for allyl isothiocyanate, a pungent-smelling compound isolated from mustard.(c) Grandisol is one component of the sex attractant of the boll weevil. Write a structural formula for grandisol given that R in the structure shown is an isopropenyl group.arrow_forwardWrite the products of the following acid-base reactions: (a) CH3OH + H2SO4 ² ? (b) CH3OH + NANH2 2 ? (c) CH3NH3+ Cl- + NaOH ?arrow_forwardWrite the reagent or draw structures of the starting material or organic product(s) in the following reactions. If more than one product is formed, identify the major product where possible. (a) (b) HO OH OH H2SO4 ? Cl₂ ? FeCl3arrow_forward
- Give reasons for the following: (i) p-nitrophenol is more acidic than p-methylphenol. (ii) Bond length of C—O bond in phenol is shorter than that in methanol. (iii) (CH3)3C—Br on reaction with sodium methoxide (Na+ _OCH3) gives alkene as the main product and not an ether.arrow_forwardGive reasons for the following :(i) Phenol is more acidic than methanol.(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (190°28′).(iii) (CH3)3C—O—CH3 on reaction with HI gives (CH3)3C—I and CH3—OH as the main products and not (CH3)3C—OH and CH3—I.arrow_forward(b) Complete the following reactions : (i) D H3C CH3 H H كما .NO2 B | Bra/Dioxane .COCH3 Aarrow_forward
- Some of the most useful compounds for organic synthesisare Grignard reagents (general formula R-MgX, where X is ahalogen), which are made by combining an alkyl halide, R-X,with Mg. They are used to change the carbon skeleton of a start-ing carbonyl compound in a reaction similar to that with R-Li:.(a) What is the product, after a final step with water, of thereaction between ethanal and the Grignard reagent of bromo-benzene? (b) What is the product, after a final step with water, ofthe reaction between 2-butanone and the Grignard reagent of2-bromopropane? (c) There are often two (or more) combina-tions of Grignard reagent and carbonyl compound that will givethe same product. Choose another pair of reactants to give theproduct in (a). (d) What carbonyl compound must react with aGrignard reagent to yield a product with the -OH group at theendof the carbon chain? (e) What Grignard reagent and carbonylcompound would you use to prepare 2-methyl-2-butanol?arrow_forwardWrite structural formulas for the following compounds (includes both old- and new-style names).(a) 2-octyne (b) ethylisopentylacetylene (c) ethynylbenzene(d) cyclohexylacetylene (e) 5-methyl-3-octyne (f) trans-3,5-dibromocyclodecynearrow_forwardIn a basic aqueous solution, esters react with hydroxide ion to form the salt of the carboxylic acid and the alcohol from which the ester is constituted. Name each of the following esters, and indicate the products of their reaction with aqueous base. (a) -OCH,CH3 (b) CH;CH2CH2-arrow_forward
