
The amount of weight percent sulfur.
Answer to Problem 79AAP
The amount of weight percent sulfur is
Explanation of Solution
Show the chemical structure bonding of polybutadiene and polystyrene as:
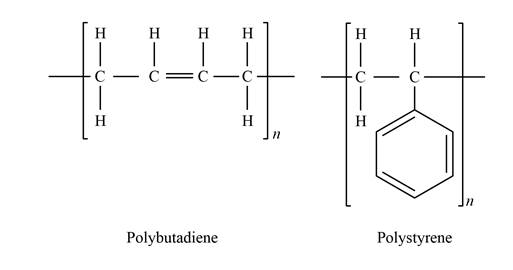
Express the weight per cent of sulfur.
Here, the molar mass of the sulfur is
Calculate the mass of sulfur at 20%.
Here, the number of mole in butadiene is
Calculate the copolymer total mass.
Here, the molar mass of the polybutadlene is
Conclusion:
The average molecular mass of the polylstyrene is
The average molecular mass of the polybutadlene is
The average molecular mass of the sulfur is
Substitute
Calculate the fraction of butadlene.
Calculate the number of moles of butadlene using 100 g of copolymer.
Substitute 1.45 g for mole butadlene and
Substitute 9.28 g for
Thus, the amount of weight percent sulfur is
Want to see more full solutions like this?
Chapter 10 Solutions
Foundations of Materials Science and Engineering
- 1 Which one below is true for thermoplastic polymers? * O They do not soften when heated and do not harden when cooled. O Most cross-linked polymers are thermoplastics. O None of them. O They are harder and stronger than thermosetting polymers.arrow_forwardBased on the facts that the free-radical polymerization of ethene is spontaneous and that polymer molecules are less disorganized than the starting monomers, decide whether the polymerization reaction is exothermic or endothermic. Explain.arrow_forwardAnswer the following questions. i) Write down the important properties of polymers. ii) What do you mean by a highly elastic material and provide an example for that material. . iii) Which type of polymer is used to manufacture plastic pipes and explain the characteristics of that polymer. (Minimum 2 points) -arrow_forward
- (b) Explain how the structural differences between the three main sub-groups of engineering polymers affect the physical and mechanical properties of the polymers. Use simple diagrams to illustrate your answer. Include examples and applications of specific polymers for each sub-group.arrow_forwardDefine the term Elastoplastic?arrow_forwardYou've been asked to select suitable candidate polymers for these applications: an ice tray with an operational temperature of -10C, a boat bumper with an operational temperature of -20C, a safety helmet, and a hairdryer with an operational temperature of 55C. Which of the below is a good choice of materials? O a. Low-density polyethylene, nylon, polypropylene, and high-density polyethylene O b. High-density polyethylene, low-density polyethylene, polypropylene, and polypropylene O c. High-density polyethylene, polypropylene, polytetrafluoroethylene, and high-density polyethylene O d. polypropylene, low-density polyethylene, poly(vinyl chloride), and polypropylene O e. Low-density polyethylene, low-density polyethylene, polytetrafluoroethylene, and high-density polyethylenearrow_forward
- ( B ) A stress of 6.7 MPa is employed to one type of a polymer under constant strain . After 40 days at 20 ° C the stress is reduced to only 8.4 MPa . When the same polymer is heated to 40 ° C the relaxation time is 40 days . 1- Calculate the relaxation time constant for this material at 25 ° C ? 2- Estimate the stress after 50 days at 25 ° C ? 3- Estimate the stress relaxation activation energy for the same polymer .arrow_forwardExplain how the structural differences between the three main sub-groups of engineering polymers affect the physical and mechanical properties of the polymers. Use simple diagrams to illustrate your answer. Include examples and applications of specific polymers for each sub-group.arrow_forwardWhat is ceiling temperature in thermodynamics of polymerization? Explain the correlation of ceiling temperature with polymers. Show the equations to support your answer.arrow_forward
- Draw the structure of the idealized modulus-temperature for thermoplastic and thermosetscurveand mention all the transitions.arrow_forwardProblem 5 Compare thermoplastic and thermosetting polymers (a) on the basis of mechanical characteristics upon heating and (b) according to possible molecular structures.arrow_forwardBriefly explain how each of the following influences the tensile or yield strength of a semicrystalline polymer and why: (a) Molecular weight (b) Degree of crystallinity (c) Deformation by drawingarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





