
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN: 9781305387102
Author: Kreith, Frank; Manglik, Raj M.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 1, Problem 1.16P
Water at a temperature of 77°C is to be evaporated slowly in a vessel. The water is in a low-pressure container surrounded by steam as shown in the sketch below. The steam is condensing at 107°C. The overall heat transfer coefficient between the water and the steam is
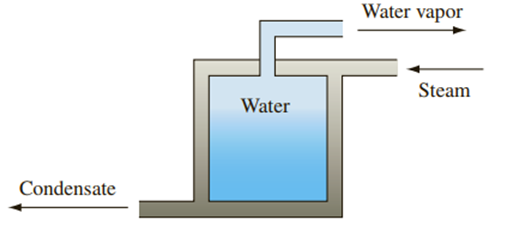
Problem 1.16
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Can someone please help me with creating an excel spreadsheet to calculate heat transfer phenomenon from a cylindrical fuel rod to the coolant?
The excel sheet needs to be able to calculate these 3 things: Heat generated in a fuel rod at a distance ‘r’ from the center; Total Heat generated in the Reactor; the maximum temperature in the fuel and the cladding surface temperature, for a fuel rod at a distance ‘r’ from the center. The fuel used in this will be UO2 and there is a thin layer of helium that will separate the fuel from the cladding material.
The user input parameters will be: the thermal neutron flux at the core, thermal conductivity of fuel, thermal conductivity of helium, thermal conductivity of the cladding, thickness of the helium layer, thickness of the cladding, diameter of the fuel pellet, fuel rod location (r), Cylindrical Reactor Size, and the fuel enrichment. For the fluid the user input will be coolant temperature and the heat transfer coefficient.
The boiling temperature of nitrogen at atmospheric pressure at sea level (1 atm) is -196°C. Therefore, nitrogen is commonly used in low temperature scientific studies since the temperature of liquid nitrogen in a tank open to the atmosphere will remain constant at -196°C until the liquid nitrogen in the tank is depleted. Any heat transfer to the tank will result in the evaporation of some liquid nitrogen, which has a heat of vaporization of 198 kJ/kg and a density of 810 kg/m3 at 1 atm. Consider a 3-m-diameter spherical tank initially filled with liquid nitrogen at 1 atm and 196°C. The tank is exposed to 22°C ambient air with a heat transfer coefficient of 22 W/m2 · °C. The temperature of the thin-shelled spherical tank is observed to be almost the same as the temperature of the nitrogen inside. Disregarding any radiation heat exchange, determine the rate of evaporation of the liquid nitrogen in the tank as a result of the heat transfer from the ambient air in kg/sec. Answer in…
A long copper cylinder 0.6 m in diameter and initially at a uniform temperature of 38oC is placed in a water
bath at 93oC. Assuming that the heat transfer coefficient between the copper and the water is 1248 W/(m2
* K), calculate the time required to heat the center of the cylinder to 66oC. As a first approximation, neglect
the temperature gradient within the cylinder; then repeat your calculation without this simplifying
assumption and compare your results.
Chapter 1 Solutions
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
Ch. 1 - 1.1 On a cold winter day, the outer surface of a...Ch. 1 - 1.2 The weight of the insulation in a spacecraft...Ch. 1 - 1.3 A furnace wall is to be constructed of brick...Ch. 1 - 1.4 To measure thermal conductivity, two similar...Ch. 1 - To determine the thermal conductivity of a...Ch. 1 - A square silicon chip 7mm7mm in size and 0.5-mm...Ch. 1 - A cooling system is to be designed for a food...Ch. 1 - 1.80 Describe and compare the modes of heat loss...Ch. 1 - Heat is transferred at a rate of 0.1 kW through...Ch. 1 - 1.10 A heat flux meter at the outer (cold) wall of...
Ch. 1 - 1.11 Calculate the heat loss through a glass...Ch. 1 - 1.12 A wall with a thickness is made of a...Ch. 1 - 1.13 If the outer air temperature in Problem is...Ch. 1 - Using Table 1.4 as a guide, prepare a similar...Ch. 1 - 1.15 A thermocouple (0.8-mm-diameter wire) used to...Ch. 1 - Water at a temperature of 77C is to be evaporated...Ch. 1 - The heat transfer rate from hot air by convection...Ch. 1 - The heat transfer coefficient for a gas flowing...Ch. 1 - 1.19 A cryogenic fluid is stored in a...Ch. 1 - A high-speed computer is located in a...Ch. 1 - 1.21 In an experimental set up in a laboratory, a...Ch. 1 - 1.22 In order to prevent frostbite to skiers on...Ch. 1 - Using the information in Problem 1.22, estimate...Ch. 1 - Two large parallel plates with surface conditions...Ch. 1 - 1.25 A spherical vessel, 0.3 m in diameter, is...Ch. 1 - 1.26 Repeat Problem 1.25 but assume that the...Ch. 1 - Determine the rate of radiant heat emission in...Ch. 1 - 1.28 The sun has a radius of and approximates a...Ch. 1 - 1.29 A spherical interplanetary probe with a 30-cm...Ch. 1 - A spherical communications satellite, 2 m in...Ch. 1 - A long wire 0.7 mm in diameter with an emissivity...Ch. 1 - Wearing layers of clothing in cold weather is...Ch. 1 - A section of a composite wall with the dimensions...Ch. 1 - A section of a composite wall with the dimensions...Ch. 1 - Repeat Problem 1.35 but assume that instead of...Ch. 1 - 1.37 Mild steel nails were driven through a solid...Ch. 1 - Prob. 1.38PCh. 1 - 1.39 On a cold winter day, the outside wall of a...Ch. 1 - As a designer working for a major electric...Ch. 1 - 1.41 A heat exchanger wall consists of a copper...Ch. 1 - 1.43 A simple solar heater consists of a flat...Ch. 1 - A composite refrigerator wall is composed of 5 cm...Ch. 1 - An electronic device that internally generates 600...Ch. 1 - 1.47 A flat roof is modeled as a flat plate...Ch. 1 - A horizontal, 3-mm-thick flat-copper plate, 1-m...Ch. 1 - 1.49 A small oven with a surface area of is...Ch. 1 - A steam pipe 200 mm in diameter passes through a...Ch. 1 - 1.51 The inner wall of a rocket motor combustion...Ch. 1 - 1.52 A flat roof of a house absorbs a solar...Ch. 1 - Determine the power requirement of a soldering...Ch. 1 - 1.54 The soldering iron tip in Problem 1.53...Ch. 1 - Prob. 1.55PCh. 1 - A pipe carrying superheated steam in a basement at...Ch. 1 - Draw the thermal circuit for heat transfer through...Ch. 1 - 1.60 Two electric resistance heaters with a 20 cm...Ch. 1 - 1.63 Liquid oxygen (LOX) for the space shuttle is...Ch. 1 - The interior wall of a large, commercial walk-in...Ch. 1 - 1.67 In beauty salons and in homes, a ubiquitous...Ch. 1 - The heat transfer coefficient between a surface...Ch. 1 - The thermal conductivity of fibreglass insulation...Ch. 1 - 1.71 The thermal conductivity of silver at 212°F...Ch. 1 - 1.72 An ice chest (see sketch) is to constructed...Ch. 1 - Estimate the R-values for a 5-cm-thick fiberglass...Ch. 1 - A manufacturer in the United States wants to sell...Ch. 1 - Referring to Problem 1.74, how many kilograms of...Ch. 1 - 1.76 Explain a fundamental characteristic that...Ch. 1 - 1.77 Explain each in your own words. (a) What is...Ch. 1 - What are the important modes of heat transfer for...Ch. 1 - 1.79 Consider the cooling of (a) a personal...Ch. 1 - Describe and compare the modes of heat loss...Ch. 1 - A person wearing a heavy parka is standing in a...Ch. 1 - Discuss the modes of heat transfer that determine...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- Determine the quantity (volume) of saline water in a steam generator. The heat energy of 1592 kJ is supplied to saline water in the steam generator to heat from 26ºC to 119ºC for the generation of water vapor, Take the density & specific heat of the solution as 1030 kg/m3 & 3.2 J/kgºK respectively. Solution: Change in Temperature (in K) Answer for part 1 Mass of the saltwater (in kg)Answer for part 2 Quantity (Volume) of saltwater (in m3) Answer for part 3arrow_forwardThe boiling temperature of nitrogen at atmospheric pressure at sea level (1 atm pressure) is -196 °C. Therefore, nitrogen is commonly used in low-temperature scientific studies since the temperature of liquid nitrogen in a tank open to the atmosphere will remain constant at -196 °C until it is depleted. Any heat transfer to the tank will result in the evaporation of some liquid nitrogen, which has a heat of vaporization of 198 kJ/kg and a density of 810 kg/m3 at 1 atm. Consider a 3-m-diameter spherical tank that is initially filled with liquid nitrogen at 1 atm and -196 °C. The tank is exposed to ambient air at 15° C, with a combined convection and radiation heat transfer coefficient of 35 W/m2⋅K. The temperature of the thin-shelled spherical tank is observed to be almost the same as the temperature of the nitrogen inside. Determine the rate of evaporation (in kg/s) of the liquid nitrogen in the tank as a result of the heat transfer from the ambient air if the tank is insulated with…arrow_forwardThe boiling temperature of oxygen at atmospheric pressure at sea level (1 atm) is -183ºC. Therefore, oxygen is used in low temperature scientific studies since the temperature of liquid oxygen in a tank open to the atmosphere remains constant at -183ºC until the liquid oxygen in the tank is depleted. Any heat transfer to the tank results in the evaporation of some liquid oxygen, which has a heat of vaporization of 213 kJ/kg and a density of 1140 kg/m3at 1 atm. Consider a 4 m diameter spherical tank initially filled with liquid oxygen at 1 atm and -183ºC. The tank is exosed to 20ºC ambient ait with a heat transfer coefficient of 25 W/m2. ºC. The temperature of the thin-shelled spherical tank is observed to be almost the same as the temperature of the oxygen inside. Disregarding any radiation heat exchange, determine the rate of evaporation of the liquid oxygen in the tank as a result of the heat transfer from the ambient airarrow_forward
- Heat Transfer Problem 1arrow_forwardQ1 a) What is the physical significance of Reynolds number? Write down 5 (five) factors that influence the convection heat transfer rates. b) Water is to be heated from 10°C to 80°C as it flows through a 2-cm-internal-diameter, 7-m- long tube. The tube is equipped with an electric resistance heater, which provides uniform heating throughout the surface of the tube. The outer surface of the heater is well insulated, so that in steady operation all the heat from the heater is transferred to the water in the tube. If the system is to provide hot water at a rate of 8 L/min, determine the convection heat transfer coefficient and temperature at the inner surface of the pipe at the exit. Q2 a) Consider heat transfer to oil flow inside a copper pipe. Is the pipe length affecting the heat transfer rate into the oil? Briefly explain. b) Used engine oil can be recycled by a patented reprocessing system. Suppose that such a system includes a process during which engine oil flows through a…arrow_forwardSteam condenses at 100°C on the outer surface of a pipe with a thermal conductivity of 180 J/ms°C. The surface heat transfer coefficient of the water flowing in the pipe is 4000 J/m²s°C, and the heat transfer coefficient created by the steam condensing outside is 10000 J/m²s°C. The length of the pipe is 5 m and the thread diameter is 25 mm. Since the pipe thickness is 1 mm, calculate the total heat transfer coefficient and the rate of heat transfer from the condensed steam to the water at 15 °Carrow_forward
- Thank you so much! I have one last follow up question. It is about heat transfer rate. Can you answer it too? It is shown in the screenshot:arrow_forward1. The operations of the industrial management plant where you work asks you to determine the length of a 2.0 cm diameter pipe that will be used to heat water from an inlet temperature of 20.0°C to an outlet temperature of 60.0 °C The operational flow of water in the pipe is to be 0.6 m/s, and the surface temperature of the wall is to be kept constant at 90.0°C by condensing steam. For the development of this task, you must obtain the properties of water at the average temperature of the fluid. To determine the forced convection heat transfer coefficient use the correlation: Nu = 0.023Re0.8p₁.0.4arrow_forwardWe have a system made up of a glass container (glass) whose bottom and top have been insulated. A mass of liquid (m) is introduced inside it at a temperature T. As this mass of water transmits heat, its temperature TI decreases, without changing the ambient temperature TA. Measurements of temperature are made with water and with saline water at different times. How would you calculate the convective heat transfer coefficient (h)?arrow_forward
- A 15 lb. block of brass with a specific heat of 0.089 is heated to 189°C and the immersed in 5 gal. of water at 66°C. What is the final temperature of the brass and water? Assume there is no heat loss.arrow_forwarda 2cm diameter copper meltallic element at an initial uniform temperature of 60 C is submerged in 20c water. The temp of the sphere after 20 seconds is 31c. Determine the average heat transfer coefficient. write assumptions.arrow_forwardDuring daytime, the outside air temperature is measured at Ta = 35 °C. A house with an inside temperature of Tin = 20 °C is separated from the outside by a brick wall as shown in Figure 1. i Briefly discuss what will happen in terms of heat transfer process and the mode of heat transfer involved. ii. Identify the parameters that affect the rate of heat transfer in this case T. = T = 35°C 20°C Brick wallarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning

Principles of Heat Transfer (Activate Learning wi...
Mechanical Engineering
ISBN:9781305387102
Author:Kreith, Frank; Manglik, Raj M.
Publisher:Cengage Learning
First Law of Thermodynamics, Basic Introduction - Internal Energy, Heat and Work - Chemistry; Author: The Organic Chemistry Tutor;https://www.youtube.com/watch?v=NyOYW07-L5g;License: Standard youtube license