
(a)
Interpretation: The hydroboration-oxidation reaction product of alkene is to be interpreted.
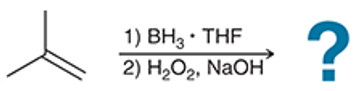
Concept introduction:
Addition reactions are the
(b)
Interpretation: The hydroboration-oxidation reaction product of alkene is to be interpreted.
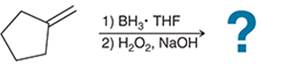
Concept introduction:
Addition reactions are the chemical reactions that show the addition of a certain group or molecule to the unsaturated carbon atoms. Alkenes are the unsaturated hydrocarbon that contains at least one double bond between the carbon atoms. Therefore, they tend to give addition reactions. Some examples of addition reactions are acid-catalyzed hydration, hydroboration-oxidation, oxymercuration-demercuration, etc.
(c)
Interpretation: The hydroboration-oxidation reaction product of alkene is to be interpreted.
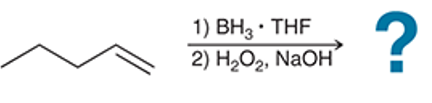
Concept introduction:
Addition reactions are the chemical reactions that show the addition of a certain group or molecule to the unsaturated carbon atoms. Alkenes are the unsaturated hydrocarbon that contains at least one double bond between the carbon atoms. Therefore, they tend to give addition reactions. Some examples of addition reactions are acid-catalyzed hydration, hydroboration-oxidation, oxymercuration-demercuration, etc.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
- Give answer all questions with explanationarrow_forwardDescribe the species that would result from the subsequent transfer of the proton from the metal to one of the Cp rings of ferrocene. Give the formal oxidation state of the metal centre and the valence electron countarrow_forwardChemistry Assuming that the reaction of E,E-2,4-hexadien-1-ol with maleic anhydride falls in the“normal electron demand” classification, illustrate the molecular orbitals involved in this reaction and how they will react.arrow_forward
- What products are expected from the oxidation with hot KMnO4 (a) solution of (a) PGE1, and (b) PGE1α?arrow_forward(a) What is the product of the following reaction? What is the catalytically active Pd species, and how is it formed? Draw a catalytic cycle, explaining what type of reaction is occurring at each stage. Indicate the formal oxidation state of Pd for each intermediate in the catalytic cycle, state how many valence electrons each intermediate has, and indicate the geometry of each Pd complex. Why is LiCl necessary? OTf OMe + Me. SnBu3 cat. PdCl₂(PPh3)2 NEt3, LICI, THEarrow_forward10arrow_forward
- Predict the major product of the following reactions..Include stereochemistry where appropriate – for all chiral products, indicate whether they are racemic or nonracemic.arrow_forwardA ketone reacts with hydrazine to form hydrazone. The reduction of hydrazone under basic conditions accompnied by the release of nitrogen gas is called Wittig Reduction O Wolff-Kishner Reduction Baeyer-Villiger Reduction O Raney Nickel Reductionarrow_forwardConsider a hypothetical chemical reaction between compound A and compound B, which produces compound C as the final product. The reaction is known to be exothermic and spontaneous. However, when the reaction is carried out under certain conditions, it fails to occur. Explain this observation and propose a potential solution to overcome this hurdle.arrow_forward
- Further substitution of the acetylferrocene ortho to the acetyl group (X), with group Y produces (n'-CsHs)Fe(n°-CsH3XY) derivatives; what is unusual about this system?arrow_forwardProvide the structures of the product or products where appropriate for the reactions shown below.arrow_forwardA chemist is attempting to synthesize a complex natural product with a highly strained cyclohexene ring system. Which type of reactants would be most suitable for achieving this goal, and why? Provide a detailed explanation of the choice of reactants and the expected outcome in terms of the Diels-Alder reaction.arrow_forward

 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


