
Concept explainers
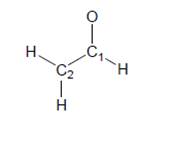
Complete each Lewis structure, draw all important resonance structures, predict a value for thebond angles requested, and explain your reasoning.
a. Nitrous acid
b. Enolate ion
Want to see the full answer?
Check out a sample textbook solution
Chapter 5 Solutions
Organic Chemistry: A Guided Inquiry
- Acrylamide, H2C=CHCONH2, is a known neurotoxin and possible carcinogen. It was a shock to all consumers of potato chips and french fries a few years ago when it was found to occur in those products. (a) Sketch the molecular structure of acrylamide and identify all bond angles. (b) Indicate which carbon-carbon bond is the stronger of the two. (c) Is the molecule polar or nonpolar? (d) The amount of acrylamide found in potato chips is 1.7 mg/kg. If a serving of potato chips is 28 g, how many moles of acrylamide are you consuming?arrow_forwardThe chemistry of the nitrite ion and HNO2: (a) Two resonance structures are possible for NO2. Draw these structures, and then find the formal charge on each atom in each resonance structure. (b) In forming the acid HNO2 an H+ ion attaches to the O atom and not the N atom of NO2. Explain why you would predict this result. (c) Two resonance structures are possible for HNO2. Draw these structures, and then find the formal charge on each atom in each resonance structure. Is either of these structures strongly preferred over the other?arrow_forwardThe electrostatic potential surface for SOCl2 is pictured here. (a) Draw a Lewis electron dot picture for the molecule, and give the formal charge of each atom. (b) What is the molecular geometry of SOCl2? Is it polar?arrow_forward
- Molecules in space: (a) In addition to molecules such as CO, HCl, H2O, and NH3, glycolaldehyde has been detected in outer space. Is the molecule polar? (b) Where do the positive and negative charges lie in the molecule? (c) One molecule found in the 1995 Hale-Bopp comet is HC3N. Suggest a structure for this molecule.arrow_forwardBond Enthalpy When atoms of the hypothetical element X are placed together, they rapidly undergo reaction to form the X2 molecule: X(g)+X(g)X2(g) a Would you predict that this reaction is exothermic or endothermic? Explain. b Is the bond enthalpy of X2 a positive or a negative quantity? Why? c Suppose H for the reaction is 500 kJ/mol. Estimate the bond enthalpy of the X2 molecule. d Another hypothetical molecular compound, Y2(g), has a bond enthalpy of 750 kJ/mol, and the molecular compound XY(g) has a bond enthalpy of 1500 kJ/mol. Using bond enthalpy information, calculate H for the following reaction. X2(g)+Y2(g)2XY(g) e Given the following information, as well as the information previously presented, predict whether or not the hypothetical ionic compound AX is likely to form. In this compound, A forms the A+ cation, and X forms the X anion. Be sure to justify your answer. Reaction: A(g)+12X2(g)AX(s)The first ionization energy of A(g) is 400 kJ/mol. The electron affinity of X(g) is 525 kJ/mol. The lattice energy of AX(s) is 100 kJ/mol. f If you predicted that no ionic compound would form from the reaction in Part e, what minimum amount of AX(s) lattice energy might lead to compound formation?arrow_forwardEach compound contains both ions and covalent bonds. Draw the Lewis structure for each compound. Show with dashes which are covalent bonds and show with charges which are ions. (a) Sodium methoxide, CH3ONa (b) Ammonium chloride, NH4Cl (c) Sodium bicarbonate, NaHCO3 (d) Sodium borohydride, NaBH4 (e) Lithium aluminum hydride, LiAlH4arrow_forward
- Draw Lewis structure(s) for the carbonate lon (CO₂). If there are equivalent resonance structures, draw all of them. n D co₂2: 0 . Draw one structure per sketcher box, and separate added sketcher boxes with the symbol. Do not include overall lon charges or formal charges in your drawing. Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. ● 6 # H Ⓒ CH, CHO: 0 Y Chemic b Draw Lewis structure(s) for the acetaldehyde molecule (CH₂CHO). If there are equivalent resonance structures, draw all of the POLICE 81 MEDITE HARA (4) Y Draw one structure per sketcher box, and separate added sketcher boxes with the symbol. Do not include overall ion charges or formal charges in your drawing. Do not draw double bonds to oxygen unless they are needed in order for the central atom to obey the octet rule. ARQQA 000-ZIF www HEADING Chartlkoodn MES DE A V Ja remove 000-n [ MacBook Airarrow_forwardConsider the anion PF52-. Draw the correct 3-D Lewis Structure. Include all resonance hybrids, if needed. If you use formal charge to determine the best structure, show your calculations. Label (with a value) the F-P-F bond angle(s). Label partial charges on atoms and polar bond vectors, if appropriate. Record the Electron Cloud Geometry and the Molecular Geometry. If the molecule/polyatomic ion has overall molecular polarity, clearly label the overall partial charges and overall molecular polarity vector on the Lewis structure.arrow_forwardAnswer the questions based on the resonance structures of S2O32– (thiosulfate) below. See attatched photo for more information. Draw an “X” through the resonance structure that is not chemically possible. On the remaining three resonance structures, write in any non-zero formal charges. Of the remaining three structures, circle the structure that will contribute the least to the resonance hybrid. Draw a resonance hybrid (including partial “δ” and full charges) for S2O32–.arrow_forward
- 10.) The structural formula of a certain aldehyde (related to formaldehyde) is H3C-CH2-CHO. Draw a Lewis structure for this aldehyde and determine the number of bonds present. Note that a single or a double or a triple bond counts as one bond. Write the number, not the word.arrow_forwardAn incomplete Lewis structure is shown below. The structure only shows the atoms and how they are connected. The molecule has a net charge of +1. H H N H Complete the Lewis structure giving all atoms full octets. If there is more than one way to do this, draw resonance structures showing all possibilities. If not, just draw one Lewis structure. Be sure to write in any non-zero formal charges. Click and drag to start drawing a structure. olo Ar 6arrow_forwardAnswer the questions in the table below about the shape of the phosphorus trifluoride (PF3) molecule. How many electron groups are around the central phosphorus atom? Note: one "electron group" means one lone pair, one single bond, one double bond, or one triple bond. What phrase best describes the arrangement of these electron groups around the central phosphorus atom? (You may need to use the scrollbar to see all the choices.) (choose one) X Śarrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





