
Concept explainers
For each
Interpretation:
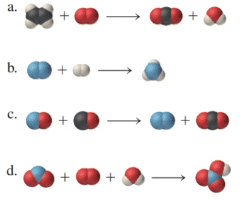
The chemical reactions are to be balanced for the given molecular model reactions, where carbon atoms are black, hydrogen atoms are white, oxygen atoms are red, and nitrogen atoms are blue.
Concept Introduction:
A chemical equation is considered to be the symbolic depiction of a chemical reaction, which contains symbols and formula.
In a chemical reaction, the starting substances on the left side are known as reactants, while the substances on the right side are known as products.
The chemical formula represents the precise number of atoms in the molecule, but not their structural arrangement
The molecular model represents the molecules in a three-dimensional configuration.
Answer to Problem 63E
Solution: The chemical formulas are represented as follows: a)
Explanation of Solution
a)
In the given molecular model, the reaction can be represented in a chemical reaction as follows:
In the next step, the
Now, in the final step, the
b)
In the given molecular model, the reaction can be represented in a chemical reaction as follows:
In the next step, the
c)
In the given molecular model, the reaction can be represented in a chemical reaction as follows:
In next step, the
Now, in the final step, the
d)
In the given molecular model, the reaction can be represented in a chemical reaction as follows:
In the next step,
Now, in the final step, the
Want to see more full solutions like this?
Chapter 4 Solutions
Chemistry In Focus
- The age of the universe is unknown, but some conclude from measuring Hubbles constant that the age is about 18 billion years old, which is about four times the age of Earth. If so, calculate the age of the universe in seconds. If you had a sample of carbon with the same number of carbon atoms as there have been seconds since the universe began, determine whether you could measure this sample on a laboratory balance that can detect masses as small as 0.1 mg.arrow_forwardReference Section 5-2 to find the atomic masses of 12C and 13C, the relative abundance of 12C and 13C in natural carbon, and the average mass (in u) of a carbon atom. If you had a sample of natural carbon containing exactly 10,000 atoms, determine the number of 12C and 13C atoms present. What would be the average mass (in u) and the total mass (in u) of the carbon atoms in this 10,000-atom sample? If you had a sample of natural carbon containing 6.0221 1023 atoms, determine the number of 12C and 13C atoms present What would be the average mass (in u) and the total mass (in u) of this 6.0221 1023 atom sample? Given that 1 g = 6.0221 1023 u, what is the total mass of I mole of natural carbon in units of grams?arrow_forward3.116 The simplest approximate chemical formula for the human body could be written as C728H4850O1970N104Ca24P16K4S4Na3Cl2Mg. Based on this formula, describe how you would rank by mass the ten most abundant elements in the human body.arrow_forward
- The formula of water is If-O. Which of the following is indicated by this formula? Explain your answer. a. The mass of hydrogen is twice that of oxygen in each molecule. b. There are two hydrogen atoms and one oxygen atom per water molecule. c. The mass of oxygen is twice that of hydrogen in each molecule. d. There are two oxygen atoms and one hydrogen atom per water molecule.arrow_forwardMass spectrometric analysis showed that there are four isotopes of an unknown element having the following masses and abundances: Three elements in the periodic table that have atomic weights near these values are lanthanum (La), atomic number 57, atomic weight 138.9055; cerium (Ce), atomic number 58, atomic weight 140.115; and praseodymium (Pr), atomic number 59, atomic weight 140.9076. Using the data above, calculate the atomic weight, and identify the element if possible.arrow_forwardThe isotope of an unknown element, X, has a mass number of 79. The most stable ion of the isotope has 36 electrons and forms a binary compound with sodium, having a formula of Na2X. Which of the following statements is(are) true? For the false statements, correct them. a. The binary compound formed between X and fluorine will be a covalent compound. b. The isotope of X contains 38 protons. c. The isotope of X contains 41 neutrons. d. The identity of X is strontium, Sr.arrow_forward
- Which of the following is true about an individual atom? Explain. a. An individual atom should be considered to be a solid. b.An individual atom should be considered to be a liquid. c. An individual atom should be considered to be a gas. d. The state of the atom depends on which element it is. e. An individual atom cannot be considered to be a solid, liquid, or gas. Justify your choice, and for choices you did not pick, explain what is wrong with them.arrow_forwardLet’s say you were doing an experiment on two different chemicals in two different test tubes. One chemical is giving off oxygen gas or hydrogen gas the other is giving off carbon dioxide gas. What one test could you do in each test tube that would prove which is giving off the oxygen or hydrogen and which is giving off the carbon dioxide?arrow_forwardCreate a diagram to trace the development of the modern periodic table based on the observations on the properties of the elements? 3. Is the periodic table useful to you as a student? Justify your answer. 2. If you were to create a simple product using metals, nonmetals, and metalloids, what would your product be like? Sketch the design and composition of your product. Then, briefly explain what properties of each material are utilized in each part of the product. 4.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co





