
Tutorials in Introductory Physics
1st Edition
ISBN: 9780130970695
Author: Peter S. Shaffer, Lillian C. McDermott
Publisher: Addison Wesley
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 27.2, Problem 1aTH
To determine
To Describe: Whether, an ideal gas process satisfies the given conditions or not.
Expert Solution & Answer
Explanation of Solution
Introduction:
The process with constant temperature and with some
Take an example of piston cylinder arrangement with an ideal gas. Gas is allowed to expand and contract. The heat is supplied or extract from the cylinder . This expands or contracts the piston slowly and produce work without change in temperature.
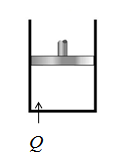
Conclusion:
Yes, in isothermal process the temperature is constant and heat is transferred.
Want to see more full solutions like this?
Subscribe now to access step-by-step solutions to millions of textbook problems written by subject matter experts!
Students have asked these similar questions
A gas with an initial temperature of 900°C undergoes the process as shown.a. What type of process is this?b. What is the final temperature?c. How many moles of gas are there?
In the process illustrated by the pV diagram in the image, the temperature of the ideal gas remains constant at 136 ∘C . Part A: How many moles of gas are involved? Part B: What volume does this gas occupy at a? (express in cubic meters) Part C: How much work was done by or on the gas from a to b? and by how much did the internal energy of the gas change during this process?
A monatomic ideal gas undergoes the thermodynamic process shown in the PV diagram at the right.
Determine whether each of the values ΔU, Q, and W for the gas is positive, negative, or zero.
*please show all work for understanding
Chapter 27 Solutions
Tutorials in Introductory Physics
Ch. 27.1 - Prob. 1aTHCh. 27.1 - In this process, which of the quantities P, V, n,...Ch. 27.1 - Consider the following incorrect student...Ch. 27.1 - Explain why it is not possible to use the ideal...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - A long pin is used to hold the piston in place as...Ch. 27.1 - Prob. 2cTHCh. 27.2 - Prob. 1aTHCh. 27.2 - Prob. 1bTHCh. 27.2 - Prob. 1cTH
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- First Law of Thermodynamics PROCEDURES: in the pictures attached A.When gas expands, Is the (W) work done by the system or (W) work done on the system? Explain your answer. B.Compare the temperature outside (surroundings) and inside (system) the bottle before pouring the boiling water into the bucket. C.Compare the temperature outside (surroundings) and inside (system) the bottle after pouring the boiling water into the bucket.arrow_forwarda. Determine the work done ON a fluid that expands from 1 to 4 as indicated in the figure at the right.b. How much work is done ON the fluid if it is compressed from 4 to 1 along the same path? * For part (a), provide a derivation equation in terms of variables for initial andfinal pressure and volume.arrow_forward0.0040 mol of gas undergoes the process as shown.a. What type of process is this?b. What are the initial and final temperatures?arrow_forward
- Answer the following questions: a.How will you explain the first law of thermodynamics? Give a specific example. b.What law explains the mechanism of heat engines? Support your answer. c. Why do machines are not 100% efficient?arrow_forwardA student is asked to sketch a pV diagram for a gas that goes through a cycle consisting of (a) an isobaric expansion, (b) a constant-volume reduction intemperature, and (c) an isothermal process that returns the gas to its initial state. The student draws the diagram as shown. What, if anything, is wrong with the student’s diagram?arrow_forwardThe change in pressure and volume of an enclosed gas taken from state A to state B at constant temperature is shown in the graph below (picture included). Was work done on the gas or by the gas? If the internal energy of the gas stayed the same during this process, did the gas absorb or release thermal energy? Explain your reasoning.arrow_forward
- Suppose a monatomic ideal gas is changed from state A to state D by one of the processes shown on the PV diagram. 1. The gas follows the constant-temperature path AC followed by the constant-pressure path CD. What is the total work done on the gas ? What is the total change in internal energy of the gas during the entire process? What is the total heat flow into the gas?arrow_forwardGive an equation for the infinitesimal change in entropy, ??, of a system in terms of the heat transfer ??? and the temperature ? at which the heat transfer occurs. Explain the sign convention.ii. A litre of water at 20 °C is placed in a fridge at 5 °C. Calculate the change in entropy of the water, including the sign, once all the water has come into thermal equilibrium.iii. Calculate the change in entropy of the fridge from part ii, including the sign.iv. Demonstrate that the Second Law has been obeyed in the process described in ii and iii.arrow_forwardPlease help me with these questions a. What is the name given to this cycle, and why is it important? The volume of the ideal gas at point 1 is V1, etc. By analysing the adiabatic processes, we find that: V_2/V_1 = V_4/V_3 b. The work done during the isothermal processes is WC (between points 1 and 2) and WH (between points 3 and 4). Find an expression for the fraction WC / WH in terms of TC and TH. c. Apply the First Law of Thermodynamics to the isothermal processes to show that the efficiency of this cycle is (please see the image with the corresponding formula for question c) d. Use the Second Law of Thermodynamics to explain why no heat engine can be more efficient than a perfectly reversible engine. You may wish to include a heat flow diagram in your answer.arrow_forward
- Table Q3 is given to collect the temperature of hot and cold water at the inlet and outlet positions in the laboratory using Tube Heat Exchanger (TD360a) by varying the cold-water flow rate to investigate the effect of cold-water flow rate on the heat exchanger’s performance. (a) Complete all the output parameters indicated in the table given in Appendix 1. (b) Draw the temperature (TH1, TH2, TC1 and TC2) on the vertical vs position (1, 2) on the horizontal axis for each flow and discuss the effect of cold water flow rate change on the exit temperature of both cold water and hot water. (c) Draw the graph of Energy Balance Coefficient and Mean Temperature Efficiency on vertical axis and cold-water flow rate on horizontal axis. Discuss the effect of flow rate on the Energy Balance Coefficient and Mean Temperature Efficiency based on your finding.arrow_forwardA gas with an initial temperature of 740 °C undergoes the process shown in. (B) What is the final temperature? (C) How many moles of gas are there?arrow_forwardConsider a thermal engine filled with N molecules of a monatomic ideal gas. The gas undergoes a cyclic transformation, as shown. (Figure 1) Processes AB and CD are isothermal (constant temperature), and processes BC and DA are isochoric (constant volume). The quantities p1, p2, V1, and V2 are defined in the figure. For example, when the gas is in state A, it has pressure p2 and volume V1; when the gas is in state B, it has volume V2, but the pressure is not given. 1. What is the change in internal energy UB−UA during the process AB? What is the change in internal energy UD−UC during the process CD? Express the change in internal energies in terms of p1, p2, V1, and/or V2. Separate your answers with a comma. 2. What is the change in internal energy UC−UB during the process BC? What is the change in internal energy UA−UD during the process DA? Express the change in internal energies in terms of p1, p2, V1, and/or V2. Separate your answers with a comma. 3. What is the total change…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning

Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning