
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
8th Edition
ISBN: 9780134015187
Author: John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Question
Chapter 2.4, Problem 2.5P
Interpretation Introduction
Interpretation:
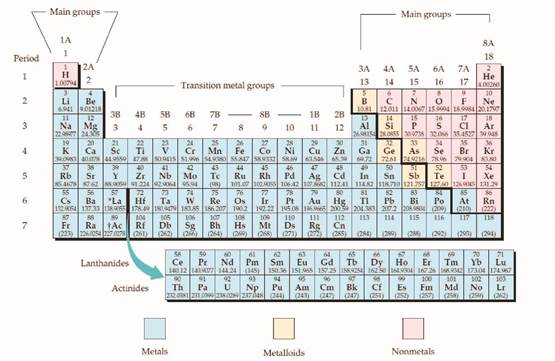
The group and period number should be given for the aluminum.
Concept introduction:
The periodic table is given below,
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Write the nuclear equation for the beta decay of Bi-214.
Compound A has molecular formula C7H7X. Its 1H-NMR spectrum shows a singlet at 2.25 ppm and two doublets, one at 7.28 ppm and one at 7.39 ppm. The singlet has an integral of three and the doublets each have an integral of two. The mass spectrum of A shows a peak at m/z = 126 and another peak at m/z = 128; the relative height of the two peaks is 3:1 respectively.
Identify what atom X is, explaining your reasoning and identify Compound A, explaining your reasoning.
2.37
Calculate the atomic weight of lithium on the
basis of the following percent composition and
atomic weights of the naturally occurring
isotopes. [Give your answer to 4
decimal places]
lithium-6-7.42% (6.0151 u)
lithium-7=92.58% (7.0160 u)
Chapter 2 Solutions
Fundamentals of General, Organic, and Biological Chemistry (8th Edition)
Ch. 2.1 - Prob. 2.1CIAPCh. 2.1 - For the Kanji character in the lower portion of...Ch. 2.2 - Use the list inside the front cover to identify...Ch. 2.3 - Prob. 2.2PCh. 2.3 - Prob. 2.3PCh. 2.3 - Prob. 2.4PCh. 2.4 - Prob. 2.5PCh. 2.4 - Prob. 2.6PCh. 2.4 - Prob. 2.7PCh. 2.4 - Prob. 2.8P
Ch. 2.4 - Prob. 2.9PCh. 2.5 - Prob. 2.10PCh. 2.5 - Prob. 2.11PCh. 2.5 - Prob. 2.12PCh. 2.5 - Prob. 2.13KCPCh. 2.5 - Prob. 2.3CIAPCh. 2.5 - Prob. 2.4CIAPCh. 2.6 - Prob. 2.14PCh. 2.7 - Prob. 2.15PCh. 2.7 - Write electron configurations for the following...Ch. 2.7 - Prob. 2.17PCh. 2.7 - Identify the atom with the following...Ch. 2.8 - Prob. 2.19PCh. 2.8 - Prob. 2.20PCh. 2.8 - Prob. 2.21PCh. 2.8 - Prob. 2.22KCPCh. 2.9 - Prob. 2.23PCh. 2.9 - Write electron-dot symbols for radon, lead, xenon,...Ch. 2.9 - Prob. 2.25PCh. 2.9 - Prob. 2.5CIAPCh. 2.9 - Prob. 2.6CIAPCh. 2 - Where on the following outline of a periodic table...Ch. 2 - Is the element marked in red on the following...Ch. 2 - Prob. 2.28UKCCh. 2 - What atom has the following orbital-filling...Ch. 2 - Use the following orbital-filling diagram to show...Ch. 2 - What four fundamental assumptions about atoms and...Ch. 2 - How do atoms of different elements differ?Ch. 2 - Prob. 2.33APCh. 2 - Prob. 2.34APCh. 2 - Prob. 2.35APCh. 2 - Prob. 2.36APCh. 2 - How many O atoms of mass 15.99 amu are in 15.99 g...Ch. 2 - Prob. 2.38APCh. 2 - What are the names of the three subatomic...Ch. 2 - Prob. 2.40APCh. 2 - Prob. 2.41APCh. 2 - Prob. 2.42APCh. 2 - Which of the following symbols represent isotopes...Ch. 2 - Prob. 2.44APCh. 2 - Prob. 2.45APCh. 2 - Prob. 2.46APCh. 2 - One of the most widely used isotopes in medical...Ch. 2 - Prob. 2.48APCh. 2 - Prob. 2.49APCh. 2 - Prob. 2.50APCh. 2 - Prob. 2.51APCh. 2 - Prob. 2.52APCh. 2 - Prob. 2.53APCh. 2 - Prob. 2.54APCh. 2 - Prob. 2.55APCh. 2 - For (a) rubidium (b) tungsten, (c) germanium, and...Ch. 2 - For (a) calcium, (b) palladium, (c) carbon, and...Ch. 2 - Prob. 2.58APCh. 2 - Prob. 2.59APCh. 2 - Prob. 2.60APCh. 2 - Prob. 2.61APCh. 2 - Prob. 2.62APCh. 2 - Prob. 2.63APCh. 2 - Prob. 2.64APCh. 2 - Prob. 2.65APCh. 2 - Prob. 2.66APCh. 2 - Prob. 2.67APCh. 2 - Prob. 2.68APCh. 2 - Prob. 2.69APCh. 2 - Prob. 2.70APCh. 2 - Prob. 2.71APCh. 2 - Determine the number of unpaired electrons for...Ch. 2 - Without looking back in the text, write the...Ch. 2 - Prob. 2.74APCh. 2 - Prob. 2.75APCh. 2 - Prob. 2.76APCh. 2 - Prob. 2.77APCh. 2 - Prob. 2.78APCh. 2 - Using n for the number of the valence shell and...Ch. 2 - What elements in addition to helium make up the...Ch. 2 - Prob. 2.81CPCh. 2 - What is the atomic number of the yet-undiscovered...Ch. 2 - Give the number of electrons in each shell for...Ch. 2 - Identify the highest-energy occupied subshell in...Ch. 2 - Prob. 2.85CPCh. 2 - Prob. 2.86CPCh. 2 - Germanium, atomic number 32, is used in building...Ch. 2 - Prob. 2.88CPCh. 2 - Prob. 2.89CPCh. 2 - What is wrong with the following electron...Ch. 2 - Prob. 2.91CPCh. 2 - Prob. 2.92CPCh. 2 - Prob. 2.93CPCh. 2 - Prob. 2.94CPCh. 2 - Prob. 2.95GPCh. 2 - Prob. 2.96GPCh. 2 - Prob. 2.97GPCh. 2 - Look again at the trends illustrated in Figures...
Knowledge Booster
Similar questions
- For Be-10, find the: a.) mase defect (in grams) b.) binding energy in kilojoules per mole. mass proton= 1.00728 amu; mass neutron= 1.00867 amu; mass Be-10 = 10.013534679 amuarrow_forwardWrite the nuclear equation for the alpha decay of Po-214.arrow_forwardLook up the valence electron configuration, covalent atomic radius, effective nuclear charge, first ionization energy and Pauling electronegativity in Chapter 8 (tables are attached). Examine the above data and answer the following questions. a) Explain why some of the elements like TI and Pb on the lower left of the p block are metallic. b) Explain why some of the elements like C, Si in the center of the p block form covalent bonds. Explain why these bonds formed by the network of these elements (as studied in Chapter 25) tend to be unreactive. c) Explain why the noble Group 8A elements are highly unreactive gases. d) Explain why some elements like F, CI, Br etc, on the upper right of the p block are highly reactive nonmetals.arrow_forward
- Elemental analysis of a compound with molar mass 342.3 g/mol gives the following mass percent composition: C 42.11%, H 6.48%, O 51.41%. Find the molecular formula of the compound. Enter your answer in the space below using the following format: if the molecular formula of a compound containing elements X, Y, and Z is X2YZ3 enter your answer as X2YZ3.arrow_forwardDo a web search to identify each of the following elements>isotopes and indicate the number of neutrons, protons, and electrons in an atom of the element>isotope:arrow_forwardLook up antimony in the periodic table 1Z = 512. How many covalent bonds would you expect it to form? Based on this information, which of the following antimony compounds is covalent and which is ionic: SbCl3 or SbCl5?arrow_forward
- The atomic emission spectrum for a particular element includes blue-violet light with wavelength 440. nm. Calculate the energy in joules of this light given that E = h c/A, and h = 6.63 x 10-34Js, and c = 3.00 x 10°m/s. (h andc are constants, A is wavelength, convert nm into m) h c E =arrow_forwardElement Z forms an ion Z 3+, which contains 31 protons. What is the identity of Z, and how many electrons does Z3+have?arrow_forwardAnother major use of bismuth has been as an ingredient in low-melting metal alloys, such as those used in firesprinkler systems and in typesetting. The element itself is a brittle white crystalline solid. How do these characteristicsfit with the fact that bismuth is in the same periodic group with such nonmetallic elements as nitrogenand phosphorus?arrow_forward
- Iodine has 37 known isotopes. Therefore, the atomic mass has a range of 108-144 amu. Which of the following statements concerning iodine is correct? A) The isotopes of iodine have between 55 and 91 protons. B) An atom of iodine can have between 55 and 91 neutrons. C) The isotopes of iodine will always have the same number of neutrons, but the protons can vary. D) The isotopes of iodine have between 108 and 144 neutrons, but the number of protons will not vary.arrow_forwardIn 1895 a student prepared three coordination compounds of chromium with the same formula CrCl2(H2O). The table below gives the color of each compound along with the number of CI ions in solution per formula unit of the compound. Complete the table by filling in the modern formula for each compound. Metal chromium has a coordination number of 6 in these compounds and an oxidation state of +2. Compound (a) Color Bright blue (b) Light green (c) Yellow Part 1 of 3 Modern formula for (a): х G CI Ions in Solutions per Formula Unit 2 1 0 00 olaarrow_forwardDerive the Henderson – Hasselbach Equation. [H*][A¯] Ка— [НА] Molecular Weight: H = 1 g/mol C= 12 g/mol N=14 g/mol O = 16 g/mol Cl= 35.45 g/molarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning
Principles Of Radiographic Imaging: An Art And A ...Health & NutritionISBN:9781337711067Author:Richard R. Carlton, Arlene M. Adler, Vesna BalacPublisher:Cengage Learning


Principles Of Radiographic Imaging: An Art And A ...
Health & Nutrition
ISBN:9781337711067
Author:Richard R. Carlton, Arlene M. Adler, Vesna Balac
Publisher:Cengage Learning
