
Concept explainers
Interpretation:
The IUPAC name of the organic compounds should be given.
Concept introduction:
Organic compounds are named systematically by using IUPAC rules.
Name of the organic compounds are given according to the number of carbon present in the molecule for example
A molecule having one carbon atom, the molecule name will start with meth etc.

If any halogens are present in the molecule, the name of the halogens as follows.

Naming the substituted
- (1) Name the parent alkane (long alkyl chain)
- (2) Number the carbon
- (3) Name and number the substituent
If the molecules have the multiple substituents, the compound named as di, tri, tetra, penta, ect.
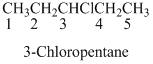
If the molecules having
The given compound is an alcohol
Example is given below
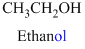

The given compound is an acid (
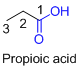 ssw
ssw
The amides are derivatives of acids and it is named as the ending of alkane with amide.
For example
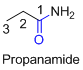
If the molecule is ester,
Esters end with “ate”
Example
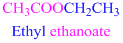
The given compound is an
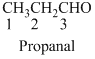
The given compound is a
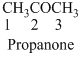
The given compound is an
Want to see the full answer?
Check out a sample textbook solution
Chapter 23 Solutions
Chemistry: Atoms First
- Name the family to which each organic compound belongs. The first answer has been filled in for you. compound CH₂ || CH₂-C-0-C- CH₂ O || CH₂ || CH–C—NH, CH, OH I | CH₂-CH-C || || CH,—C–NH CH, X family ester 0arrow_forwardComplete the following molecular formula of Ethyl acrylate with C, CH, CH2, or CH3.arrow_forwardname the alkanearrow_forward
- What is the parent alkane for the following structure?arrow_forwardIllustrate the Structures for the two simplest acyclic alkanes ?arrow_forwardQuiana whose structure is shown below is a synthetic fabric that feels very much like silk. What are the structures of the two monomers that are used to make Quiana?arrow_forward
- Draw all possible structure(s) and give the IUPAC systematic name(s) of an alkane or cycloalkane with the formula C8H18 that has only primary hydrogen atoms. Draw the structure(s).arrow_forwardDescribe conformations of alkanes.arrow_forwardExplain why alkenes are much more reactive than alkanes towards chlorine (CI2) or bromine (Br2) in the dark at room temperature, and why alkanes do not react with HCI (g) or HBr (g) whereas alkenes do.arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning



