
University Physics Volume 2
18th Edition
ISBN: 9781938168161
Author: OpenStax
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 69P
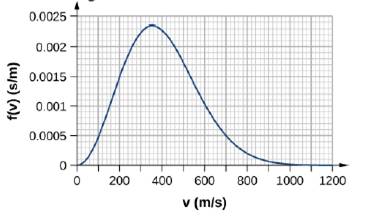
By counting squares in the following figure, estimate the fraction of argon atoms at T = 300 K that have speeds between 600 m/s and 800 m/s. The curve is correctly normalized. The value of a square is its length as measured on the x-axis times its height as measured on the y-axis, with the units given on those axes.
 `
`
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please asap
A sample of argon gas is at a pressure of 1.5 x 105 Pa and a temper-
ature of 350 K.
(a) Determine the number of argon atoms per unit volume.
(b) Estimate the speed of the argon atoms between collisions.
(c) Estimate the number of collisions that a single atom of argon
makes per second. The diameter of an argon atom is approxi-
mately 3.4 x 10-10 m.
At what height is the atmospheric pressure 29.0% of what it is at sea level? Assume
the molar mass of the air molecules to be 29.0 g/mol and that the air temperature is
uniformly 287 K.
[Answer in kilometres with 3 sig digits, but do not enter units with your answer]
Chapter 2 Solutions
University Physics Volume 2
Ch. 2 - Check Your Understanding The recommended daily...Ch. 2 - Check Understanding The density of in a Classroom...Ch. 2 - Check Your Understanding Liquids and solids have...Ch. 2 - Check Your Understanding If you consider a very...Ch. 2 - Check Your Understanding Which has a longer mean...Ch. 2 - Check Your Understanding Suppose 2 moles of helium...Ch. 2 - Two H2 molecules can react with one O2 molecule to...Ch. 2 - Under what circumstances would you expect a gas to...Ch. 2 - A constant-volume gas thermometer contains a fixed...Ch. 2 - Inflate a balloon at room temperature. Leave the...
Ch. 2 - In the last chapter, free convection was explained...Ch. 2 - How is momentum related to the pressure exerted by...Ch. 2 - If one kind of molecule has double the radius of...Ch. 2 - What is the average velocity of the air molecules...Ch. 2 - Why do the atmospheres of Jupiter, Saturn, Uranus,...Ch. 2 - Statistical mechanics says that in a gas...Ch. 2 - Which is more dangerous, a closet where tanks of...Ch. 2 - Experimentally it appears that many polyatomic...Ch. 2 - One might think that the internal energy of...Ch. 2 - You mix 5 moles of H2 at 300 K with 5 moles of He...Ch. 2 - One cylinder contains helium gas and another...Ch. 2 - Repeat the previous question if one gas is still...Ch. 2 - An ideal gas is at a temperature of 300 K. To...Ch. 2 - The gauge pressure in your car tires is...Ch. 2 - Suppose a gas-filled incandescent light bulb is...Ch. 2 - People buying food in sealed bags at high...Ch. 2 - How many moles are there in (a) 0.0500 g of N2 gas...Ch. 2 - A cubic container of volume 2.00 L holds 0.500 mol...Ch. 2 - Calculate the number of moles in the 2.00-L volume...Ch. 2 - An airplane passenger has 100 cm3 of air in his...Ch. 2 - A company advertises that it delivers helium at a...Ch. 2 - According to...Ch. 2 - An expensive vacuum system can achieve a pressure...Ch. 2 - The number density N/V of gas molecules at a...Ch. 2 - A bicycle tire contains 2.00 L of gas at an...Ch. 2 - In a common demonstration, a bottle is heated and...Ch. 2 - A high-pressure gas cylinder contains 50.0 L of...Ch. 2 - Find the number of moles in 2.00 L of gas at 35.0 ...Ch. 2 - Calculate the depth to which Avogadro's number of...Ch. 2 - (a) What is the gauge pressure in a 25.0 cc car...Ch. 2 - A person hits a tennis ball with a mass of 0.058...Ch. 2 - A person is in a closed room (a racquetball court)...Ch. 2 - Five bicyclists are riding at the following...Ch. 2 - Some incandescent light bulbs are filled with...Ch. 2 - Typical molecular speeds (vrms) are large, even at...Ch. 2 - What is the average kinetic energy in joules of...Ch. 2 - What is the ratio of the average translational...Ch. 2 - What is the total translational kinetic energy of...Ch. 2 - The product of the pressure and volume of a sample...Ch. 2 - What is the gauge pressure inside a tank of...Ch. 2 - If the rms speed of oxygen molecules inside a...Ch. 2 - The escape velocity of any object from Earth is...Ch. 2 - The escape velocity from the Moon is much smaller...Ch. 2 - Nuclear fusion, the energy solute of Sun, hydrogen...Ch. 2 - Suppose that the typical speed (vrms) of carbon...Ch. 2 - (a) Hydrogen molecules (molar mass is equal to...Ch. 2 - There are two important isotopes of uranium, U235...Ch. 2 - The partial pressure of carbon dioxide in the...Ch. 2 - Dry air consists of approximately 78% nitrogen,...Ch. 2 - (a) Using data from the previous problem, find the...Ch. 2 - (a) Given that air is 21% oxygen, find the minimum...Ch. 2 - (a) If the partial pressure of water vapor is 8.05...Ch. 2 - To give a helium atom nonzero angular momentum...Ch. 2 - (a) How much heat must be added to raise the...Ch. 2 - A sealed, rigid container of 0.560 mol of an...Ch. 2 - A sample of neon gas (Ne, molar mass M=20.2 g/mol)...Ch. 2 - A steel container of mass 135 g contains 24.0 g of...Ch. 2 - A sealed room has a volume of 24 m3. It's filled...Ch. 2 - Heliox, a mixture of helium and oxygen, is...Ch. 2 - Professional divas sometimes use heliox,...Ch. 2 - In car racing, one advantage of mixing liquid...Ch. 2 - In a sample of hydrogen sulfide ( M=34.1 g/mol) at...Ch. 2 - Using the approximation v1v1+v f(v)dvf(v1)v for...Ch. 2 - Using the method of the preceding problem,...Ch. 2 - By counting squares in the following figure,...Ch. 2 - Using a numerical integration method such as...Ch. 2 - Find (a) the most probable speed, (b) the average...Ch. 2 - Repeat the preceding problem for nitrogen...Ch. 2 - At what temperature is the average speed of carbon...Ch. 2 - The most probable speed for molecules of a gas at...Ch. 2 - a) At what temperature do oxygen molecules have...Ch. 2 - In the deep space between galaxies, the density of...Ch. 2 - (a) Find the density in SI units of air at a...Ch. 2 - The air inside a hot-air balloon has a temperature...Ch. 2 - When an air bubble rises from the bottom to the...Ch. 2 - (a) Use the ideal gas equation to estimate the...Ch. 2 - One process for decaffeinating coffee uses carbon...Ch. 2 - On a winter day when the air temperature is 0 ,...Ch. 2 - On a warm day when the air temperature is 30 , a...Ch. 2 - (a) People often think of humid air as "heavy."...Ch. 2 - The mean flee path for helium at a certain...Ch. 2 - The mean free path for methane at a temperature of...Ch. 2 - In the chapter on fluid mechanics, Bernoulli's...Ch. 2 - Find the total number of collisions between...Ch. 2 - (a) Estimate the specific heat capacity of sodium...Ch. 2 - A sealed, perfectly insulated container contains...Ch. 2 - Find the ratio f(vp)/f(vrms) for hydrogen gas (...Ch. 2 - Unreasonable results. (a) Find the temperature of...Ch. 2 - Unreasonable results. (a) Find the sped of...Ch. 2 - An airtight dispenser for drinking water is 25 cm...Ch. 2 - Eight bumper cars, each with a mass of 322 kg. are...Ch. 2 - Verify that vp=2kBTm.`Ch. 2 - Verify the normalization equation 0f(v)dv=1 In...Ch. 2 - Verify that v=8kBTm. Make the same scaling...Ch. 2 - Verify that vrms=v2=3kBTm.`
Additional Science Textbook Solutions
Find more solutions based on key concepts
Explain what happens to the energy carried by light that it is dimmed by passing it through two crossed polariz...
College Physics
Consider the two experiments described above. When the momentum of an object or system of objects did not chang...
Tutorials in Introductory Physics
81. Bronco dives from a hovering helicopter and finds his momentum increasing. Does this violate the conservati...
Conceptual Physical Science (6th Edition)
47(II) What gauge pressure in the water pipes is necessary if a fire hose is to spray water to a height of 16 m...
Physics: Principles with Applications
Where (in the southern sky, on the eastern horizon, on the western horizon, high in the sky, etc.) would you l...
Lecture- Tutorials for Introductory Astronomy
Describe the motion of the dipole shown in Fig. 21–44 if it is released from rest at the position shown.
FIGURE...
Physics for Scientists and Engineers with Modern Physics
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Please asaparrow_forwardA bubble of air is rising from the sea floor, from a depth of about 500 m. At this depth the pressure is 5.1*106 Pa (about 50.3 atm) and is at a temperature of about 7.0° C. It rises to the surface with the atmosphere, at a temperature of 20° C. If the bubble is observed to have a volume of 4 cm³ at the surface, what was its volume at the sea floor? Assume the number of moles of gas in the bubble is constant. B.arrow_forwardThe tidal lung volume of human breathing, representing the amount of air inhaled and exhaled in a normal breath, is 500 cm3. (Assume atmospheric pressure.) (a) What is the number of molecules of air inhaled with each human breath when the air temperature is 27.0°C?arrow_forward
- The equation of state of a gas can be written in the form p = nkT(1 + Bn) where p is the mean pressure of the gas, T its absolute temperature, n = N/V the number of molecules per unit volume, and B2 = B(T) is the second virial The discussion of Sec. 5-10 showed that Be is an increasing function coefficient. of the temperature. Find how the mean internal energy E of this gas dspends on its volume V, i.e., find an expression for (OE/0V)r. Is it positive or negative?arrow_forwardConsider a given volume of helium gas at room temperature (20.0 °C). [Molar mass of helium is 4.00 × 10-³ kg mol−1] (i) Calculate the average speed of a molecule of the gas. Give your answer in scientific notation and specified to an appropriate number of significant figures, in the empty box below. = m s−1 (ii) At what temperature would the average translational energy of the gas be one third of the average translational energy at room temperature? Give your answer by entering numbers, specified to an appropriate number of significant figures, into the empty box below. temperature = Karrow_forward[11] An experimental balloon contains hydrogen gas (H2) at a temperature of 300 K and a pressure of 1 atm (1.01 X 10° N / m?). (a) Calculate the mean-free path of a hydrogen molecule. Assume that a H2 molecule is effectively spherical, with a mean diameter of 1.6 X 1010 m. (b) Calculate the available volume per molecule (VI N), and find the average distance between each molecule and its nearest neighboring molecule (approximately the cube root of the available volume). Which is larger, the mean free path or the average nearest-neighbor distance between molecules? Exploring relationshipsarrow_forward
- gasarrow_forward(a) How many atoms of helium gas fill a spherical balloon of diameter 30.6 cm at 23.0°C and 1.00 atm? Enter a number lationship between pressure, volume and temperature for an ideal gas? atoms (b) What is the average kinetic energy of the helium atoms? (c) What is the ms speed of the helium atoms? km/sarrow_forwardEstimate the number of people in the world who are suffering from the common cold on any given day. (Remember that a person suffers from a cold for about a week, and assume that the average person catches a cold twice a year. The population of Earth is approximately seven billion.) O 10² O 105 O 108 O 10¹3arrow_forward
- b) A 10 m³ container contains an ideal gas and under a pressure of 3.5 atm is placed in room temperature (293 K). Assuming the volume of the container is constant, what is the pressure of gas when the temperature is increased to 308 K? (CO3, PO1, C3) c) In an internal combustion engine, air at atmospheric pressure is compressed in the cylinder by a piston to 1/9 of its original volume. Calculate the estimated pressure (in atm). (CO3, PO1, C3)arrow_forwardIf the temperature of an ideal gas is held constant, the relationship between its pressure in absolute value, and volume is O inverse O quadratic direct O parabolicarrow_forwardThe pressure in interplanetary space is estimated to be of the order of 10^-14 Pa. Calculate: A) the average number of molecules per cubic meter B) the number of molecules colliding per cubic meter per second C) the mean free path in miles. Assume thay the only hydrogen atoms are present and free temperature is 1000k. Assume molecular diameter 0.2 mm. Show complete solution, and round the final answer up to 4 decimal places.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning
Physics for Scientists and Engineers: Foundations...PhysicsISBN:9781133939146Author:Katz, Debora M.Publisher:Cengage Learning University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University
University Physics Volume 1PhysicsISBN:9781938168277Author:William Moebs, Samuel J. Ling, Jeff SannyPublisher:OpenStax - Rice University


Physics for Scientists and Engineers: Foundations...
Physics
ISBN:9781133939146
Author:Katz, Debora M.
Publisher:Cengage Learning

University Physics Volume 1
Physics
ISBN:9781938168277
Author:William Moebs, Samuel J. Ling, Jeff Sanny
Publisher:OpenStax - Rice University