
Concept explainers
What is the
a. 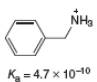
b. 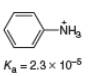
c. 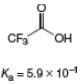
(a)
Interpretation: The value of
Concept introduction: The equilibrium constant of an acid dissociation reaction depends on the strength of an acid involved in the reaction. The
Acid dissociation constant
For an acid dissociation reaction,
The strength of an acid decreases as the value of
Answer to Problem 2.46P
The value of
Explanation of Solution
The given value of
The value of
Substitute the value of
Simplify the above equation,
Hence, the value of
The value of
(b)
Interpretation: The value of
Concept introduction: The equilibrium constant of an acid dissociation reaction depends on the strength of an acid involved in the reaction. The
Acid dissociation constant
For an acid dissociation reaction,
The strength of an acid decreases as the value of
Answer to Problem 2.46P
The value of
Explanation of Solution
The given value of
The value of
Substitute the value of
Simplify the above equation,
Hence, the value of
The value of
(c)
Interpretation: The value of
Concept introduction: The equilibrium constant of an acid dissociation reaction depends on the strength of an acid involved in the reaction. The
Acid dissociation constant
For an acid dissociation reaction,
The strength of an acid decreases as the value of
Answer to Problem 2.46P
The value of
Explanation of Solution
The given value of
The value of
Substitute the value of
Simplify the above equation.
Hence, the value of
The value of
Want to see more full solutions like this?
Chapter 2 Solutions
Organic Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





