
(a)
Interpretation:
The volume of the
Concept Introduction:
- The molarity is the number of moles of the solute dissolved per liter volume of the solution. It is represented in mathematical term such that,
Where, n is the number of moles,
V is the volume of the solution.
- The equation of dilution is given as,
Where,
(a)
Answer to Problem 41A
Explanation of Solution
Given information:
The acetic acid volume is,
The molarities of acetic acid and sodium hydroxide is,
Calculation:
The molarity formula is,
And,
- The equation of dilution is given as,
The balanced equation of the given solution is,
Hence, 1 mole of
So, Firstly, calculate the number of moles of hydrogen ion in
For neutralizing the reaction, the number of hydrogen ions must be equal to the number of hydroxide ions. The hydroxide ions are limiting.
According o the balanced equations both hydrogen and hydroxide ions are reacted in a
Therefore, the number of moles of hydroxide ions are,
Now, calculate the volume of the
The volume of the
(b)
Interpretation:
The volume of the
Concept Introduction:
- The molarity is the number of moles of the solute dissolved per liter volume of the solution. It is represented in mathematical term such that,
Where, n is the number of moles,
V is the volume of the solution.
- The equation of dilution is given as,
Where,
(b)
Answer to Problem 41A
Explanation of Solution
Given information:
The volume is,
The molarities of
Calculation:
- The molarity formula is,
And,
- The equation of dilution is given as,
The balanced equation of the given solution is,
Hence, 1 mole of
So, Firstly, calculate the number of moles of hydrogen ion in solution
For neutralizing the reaction, the number of hydrogen ions must be equal to the number of hydroxide ions. The hydroxide ions are limiting.
According to the balanced equations both hydrogen and hydroxide ions are reacted in a
Therefore, the number of moles of hydroxide ions are,
Now, calculate the volume of the
The volume of the
(c)
Interpretation:
The volume of the
Concept Introduction:
- The molarity is the number of moles of the solute dissolved per liter volume of the solution. It is represented in mathematical term such that,
Where, n is the number of moles,
V is the volume of the solution.
- The equation of dilution is given as,
Where,
(c)
Answer to Problem 41A
Explanation of Solution
Given information:
The volume is,
The molarities of
Calculation:
- The molarity formula is,
And,
- The equation of dilution is given as,
The balanced equation of the given solution is,
Hence, 1 mole of
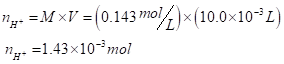
For neutralizing the reaction, the number of hydrogen ions must be equal to the number of hydroxide ions.
According to the balanced equations both hydrogen and hydroxide ions are reacted in a
Therefore, the number of moles of hydroxide ions are,
Now, calculate the volume of the
The volume of
(d)
Interpretation:
The volume of the
Concept Introduction:
- The molarity is the number of moles of the solute dissolved per liter volume of the solution. It is represented in mathematical term such that,
Where, n is the number of moles,
V is the volume of the solution.
- The equation of dilution is given as,
Where,
.
(d)
Answer to Problem 41A
Explanation of Solution
Given information:
The volume is,
The molarities of
Calculation:
- The molarity formula is,
And,
- The equation of dilution is given as,
The balanced chemical equation is,
Hence, 1 mole of Sulphuric acid is neutralizes the 2 moles of sodium hydroxide.
Firstly, calculate the number of moles of hydrogen ion in
For neutralizing the reaction, the number of hydrogen ions must be equal to the number of hydroxide ions.
According to the balanced equations both hydrogen and hydroxide ions are reacted in a
Therefore, the number of moles of hydroxide ions are,
Now, calculate the volume of the
The volume is
Chapter 15 Solutions
World of Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





