
(a)
Interpretation:
Determine the structural formula from the given molecular formula:
20 aromatic
Concept Introduction:
The spatial arrangement of atom within a compound that are joined together is identified with the help of structural formula.
Explanation of Solution
The molecular formula C7 H9 N is 20 on the
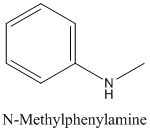
(b)
Interpretation:
Determine the structural formula from the given molecular formula:
30 aromatic amines, C8 H11 N.
Concept Introduction:
The spatial arrangement of atom within a compound that are joined together is identified with the help of structural formula.
Explanation of Solution
Tertiary amines are those compounds in which three hydrogen atoms of ammonia are replaced by alkyl or aryl groups. They are represented as R3 -N or 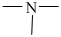
The molecular formula, C8 H11 N is 30 on the aromatic amine. Hence, it has six-membered ring. The nitrogen atom is attached to three groups in which one is an aryl group and the other two are methyl groups.
Thus, the structural formula for the molecular formula C8 H11 N is as below:
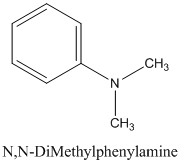
(c)
Interpretation:
Determine the structural formula from the given molecular formula:
10 aliphatic amines, C7 H9 N.
Concept Introduction:
The spatial arrangement of atom within a compound that are joined together is identified with the help of structural formula.
Explanation of Solution
Primary amines are those compounds in which one of the hydrogen atoms of ammonia is replaced by an alkyl or an aryl group. They are represented as 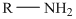
The molecular formula C7 H9 N is 10 on the aliphatic amine. Hence it has
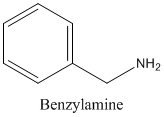
(d)
Interpretation:
Determine the structural formula from the given molecular formula:
A chiral 10 amine, C4 H11 N.
Concept Introduction:
The spatial arrangement of atom within a compound that are joined together is identified with the help of structural formula.
Explanation of Solution
Primary amines are those compounds in which one of the hydrogen atoms of ammonia is replaced by an alkyl or aryl group. They are represented as
The carbon atom attach to four different group is called a chiral atom. When a molecule possesses such atom, then they are not superimposable on their mirror image.
The molecular formula C4 H11 N is 10 on the aliphatic amine, and it is a chiral molecule. Hence, the chiral carbon atom is attached to four different groups, which include -NH2 group, the -CH3 group, the -CH2 group and the -H group. The structural formula of C4 H11 N is as below:
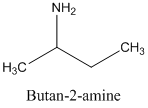
(e)
Interpretation:
Determine the structural formula from the given molecular formula:
A 30 heterocyclic amine, C5 H11 N.
Concept Introduction:
The spatial arrangement of atom within a compound that are joined together is identified with the help of structural formula.
Explanation of Solution
Tertiary amines are those compounds in which three hydrogen atoms of ammonia are replaced by alkyl or aryl groups. They are represented as R3 -N or 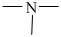
The carbon atom attach to four different group is called a chiral atom. When a molecule possesses such atom, then they are not superimposable on their mirror image.
The molecular formula C5 H11 N is 30 on the heterocyclic amine. This structure has a five membered heterocyclic ring. This ring contains four carbons and one nitrogen atom. The nitrogen atom in the ring is bonded to the methyl group. The structural formula of C4 H11 N is as below:
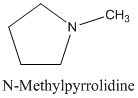
(f)
Interpretation:
Determine the structural formula from the given molecular formula:
A trisubstituted 10 aromatic amine, C9 H13 N.
Concept Introduction:
The spatial arrangement of atom within a compound that are joined together is identified with the help of structural formula.
Explanation of Solution
Primary amines are those compounds in which one of the hydrogen atoms of ammonia is replaced by an alkyl or aryl group. They are represented as
The molecular formula C9 H13 N is tri substituted 10 on the aromatic amine. Hence, it has six membered rings with three methyl groups. The structural formula of C5 H13 N is as below:
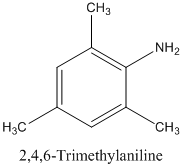
(g)
Interpretation:
Determine the structural formula from the given molecular formula:
A chiral quaternary ammonium salt, C9 H22 NCl.
Concept Introduction:
The spatial arrangement of atom within a compound that are joined together is identified with the help of structural formula.
Explanation of Solution
Quaternary amines are those compounds in which all four of the hydrogen atoms of the ammonium ion are replaced by an alkyl or aryl group. It is an ionic quaternary salt.
When the carbon atom is attached to four different groups, it is called a chiral atom. When a molecule possesses such an atom, then it is not super imposable on its mirror image.
The molecular formula C9 H22 NCl is a quaternary amine and it is a chiral molecule. Hence, the chiral nitrogen atom is attached to four different groups, which include -CH2 CH3 group, the -CH2 CH2 CH3 group, the -CH3 CHCH3 group, and the -CH3 group. Therefore, the quaternary ammonium salt of C9 H22 NCl is as below:
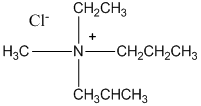
Want to see more full solutions like this?
Chapter 15 Solutions
Introduction To General, Organic, And Biochemistry
- 16-15 There are eight primary amines with the molecular formula C5H13N. (a) Name and draw a structural formula for each amine. (b > Which of these amines are chiral?arrow_forwardTRUE OR FALSE (a) There are three amines with the molecular formula C3H9N. (b) Aldehydes, ketones, carboxylic acids, and esters all contain a carbonyl group. (c) A compound with the molecular formula of C3H6O may be either an aldehyde, a ketone, or a carboxylic acid. (d) Bond angles about the carbonyl carbon of an aldehyde, a ketone, a carboxylic acid, and an ester are all approximately 109.5°. (e) The molecular formula of the smallest aldehyde is C3H6O, and that of the smallest ketone is also C3H6O. (f) The molecular formula of the smallest carboxylic acid is C2H4O2.arrow_forwardDraw the structure of a compound of molecular formula C4H,,NO that fits each description: (a) a compound that contains a 1° amine and a 3° alcohol; (b) a compound that contains a 3° amine and a 1° alcohol.arrow_forward
- Determine the IUPAC name of compound A and give the abbreviated (topological) structure of compound B:arrow_forwardDraw the structure of a compound of molecular formula C 9H 11NO that contains a benzene ring and a: (a) 1 ° amide; (b) 2 ° amide; (c) 3 ° amide.arrow_forwardGive the IUPAC name of the following compound:arrow_forward
- (a) Draw the structures for the eight constitutional isomers of molecular formula C 4H 11N. (b) Give the systematic name for each amine. (c) Identify the chirality center present in one of the amines.arrow_forwardThe following amines have the same molecular formula (C5H13N), but their boiling points are significantly different. Explain why. H `NH2 2-Methylbutan-1-amine Boiling point = 97 °C N-Methylbutan-2-amine Boiling point = 84 °C N-Ethyl-N-methylethan-1-amine Boiling point = 65 °Carrow_forwardClassify the formulas as amines, amides, or neither. Amines Amides Neither Answer Bank CH;CH,OCCHCH,CH3 RCONHR' H,C-NH, RCH,NH, CH, CH, C-NH, CH3 СООНarrow_forward
- There are eight primary amines with the molecular formula C5H13N . (a) Name and draw a structural formula for each amine. (b) Which of these amines are chiral?arrow_forwardGive the IUPAC and common names of an amine with a) 5 C atoms (b) 6 C atoms, C) 7 C atoms.arrow_forwardwhat are the reactions discussed in the Amines/Amides chapterarrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning





