
Concept explainers
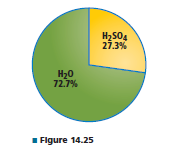
What is the mole fraction of H 2 S O 4 in a solution containingthe percentage of sulfuric acid and water shownin Figure 14.25?
Interpretation:
The mole fraction of H2SO4 in the sulfuric acid solution is to be calculated.
Concept introduction:
Mole fraction: Mole fraction defined as the number of moles of one component divided by total the number of moles in the mixture.
The mole fraction for the solute(
Answer to Problem 84A
The mole fraction of
Explanation of Solution
Given information:
The solution contains:
Let assume, the mass of the solution = 100 gm
So, the mass of H2SO4 = 27.3 gm and the mass of water = 72.7 gm
(Note: Molecular weight of sulfuric acid = 98.1 gm/mol)
(Note: Molecular weight of water = 18 gm/mol)
Chapter 14 Solutions
Chemistry: Matter and Change
Additional Science Textbook Solutions
General, Organic, and Biological Chemistry (3rd Edition)
Introductory Chemistry (6th Edition)
Chemistry: The Central Science (13th Edition)
Organic Chemistry (9th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





