
Concept explainers
(a)
Interpretation:
The compound that is more soluble in water should be determined.
CH3 OH or CH3 OCH3.
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
(b)
Interpretation:
The compound that is more soluble in water should be determined.
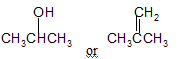
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
(c)
Interpretation:
The compound that is more soluble in water should be determined.
CH3 CH2 CH2 SH or CH3 CH2 CH2 OH.
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
(d)
Interpretation:
The compound that is more soluble in water should be determined.
CH3 CH2 Cl or NaCl.
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
(e)
Interpretation:
The compound that is more soluble in water should be determined.
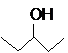 or
or 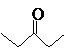
Concept Introduction:
Water solubility is known as the measurement of the chemical substance which can dissolve into the water at the specific temperature. The solubility’s unit is mg/L or ppm i.e. parts per million. Water solubility is the most vital properties which affects the environmental fate and bioavailability of the chemical substances.
Trending nowThis is a popular solution!

Chapter 14 Solutions
Introduction to General, Organic and Biochemistry
- 17-26 Account for the fact that acetone has a higher boiling point (56°C) than ethyl methyl ether (11°C) even though their molecular weights are almost the same.arrow_forward17-69 Propanal (bp 49°C) and 1-propanol (bp 97°C) have about the same molecular weight, yet their boiling points differ by almost 50°C. Explain this fact.arrow_forward16-17 Propylamine (bp 48°C), ethylmethylamine (bp 37°C), and trimethylamine (bp 3°C) are constitutional isomers with the molecular formula C3HgN. Account for the fact that trimethylamine has the lowest boiling point of the three and propylamine has the highest.arrow_forward
- PQ-15. Which reactants would NOT produce this alcohol? (A) (C) H 1) CH3MgBr 2) NHẢ 1) CH3CH₂MgBr 2) NH (B) (D) of 요 1) NaBH4 2) H3O+ 1) CH3MgBr 2) NHA OHarrow_forward12 Write the IUPAC names for these compounds. O (a) CHO CHOH (c) | CHOH I CH₂OH OH (b) (d) CHO NH₂ Oarrow_forwardONANO (a) Circle the two compounds that are not carboxylic acid derivatives. Circle only two. CONHCH3 CN =NOH NH COCI (b) Circle the two compounds that can be hydrolyzed to a ketone in aqueous acid. Circle only two. N-PH (c) Circle the two compounds that can be converted to an aldehyde by reduction using DIBAL (also known as DIBAL-H). Circle only two. .CHO он CN (d) Circle the two most reactive compounds with water. Circle only two DEt OMe NH2 CH;CH,CN (e) Circle the strongest acid. [2 marks] CO.Et CO-H NO2 CHO (1) Circle ethyl benzoate and maleic anhydride. COCH,CH, .Co.CH,CH3 (g) Circle the two compounds that can be reduced to an amine using NaBH4. Circle only two. 'NH2 CN NHarrow_forward
- CH;CH2CH,CH,CH2B1 NH3 G + H + excessarrow_forwardAnswer (d) QUESTION 13 Which molecule or ion contains the least basic nitrogen atom? (c) CH3NH2 (d) CH3 NH2 NHz (a) (cHs),N Li (b) Answer (a) O Answer (b) O Answer (c) O Answer (d) QUESTION 14 What is the final organic product for the following malonic ester synthesis? CH2-COOET (1) NaOEt, EtOH (2) Br Br (3) NaOEt, ETOH (4) H3O* COOET heatarrow_forwardWhich of the following structures and IUPAC names are incorrectly matched? * oe (A) Methyleyclohexanoate (В) `NH2 Br 4-Bromo-3-chlorohexanamide 2-Ethoxypentane (D) HOOC HOOC 3,5-Nonanedioic acid В A Darrow_forward
- When a compound like napihthalene C1oHa is dissolved in t-butyl methyl ether, then that solution is extracted with 3 M NaOH, and then the resulting basic aqueous layer is acidified with 6M HCI, what happens to the acidified aqueous layer? a) The naphthalene stays in the the aqueous layer as C10Ha O b) The naphthalene precipitates out as a solid, C10He O C) Nothing happens to the aqueous layer other than a dramatic raising of the pH d) The naphthalene stays in the aqueous layer as C10H>Na O e) Nothing happens to the aqueous layer other than a dramatic lowering of the pH O) The acid precipitates out as a solid, C10H>Naarrow_forwardWhich of the following carboxylic acids has the highest solubility in water? A) CH₃CH₂C(=O)OH B) CH₃CH₂CH₂CH₂C(=O)OH C) CH₃(CH₂)₅C(=O)OH D) CH₃(CH₂)₁₀C(=O)OH E) All of these have equal solubility.arrow_forward1;YO INO O a ".| Asiacell a 4 Asiacell -> العنوان اليوم، الساعة ۱:۰۱ ص بدون تصنيف ۲ Rank the compounds according to their increased melting point and give the reason benzoic acid ,benzophenone,sodium benzoate Aa كتابة يدوية المعرض تنسيق q'| w² ез r4 t5 y6 u7 i 8 0° a fF g h į k | £ zž x C v b m' 123 Q 1 English ► 自 國arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
