
11.35 For the reaction 2 NO(g) + 2 H?(g) — N,(g) + 2 H,O(g) at 1100°C, the following data have been obtained:
| [NOJ | [HJ | Rate = A(N2]/At |
| (mol L~1) | (mol L_1) | (mol L-1 s_1) |
| 5.0 X 10’1 | 0.32 | 0.012 |
| 1.0 X 10~’ | 0.32 | 0.048 |
| 1.0 X 10"2 | 0.64 | 0.096 |
Derive a rate law for the reaction and determine the value of the rate constant.
Interpretation: Given the experimental data obtained for a reaction at
Concept Introduction: Orders of reaction are constantly determined by doing experiments. Consequently without experimental information, we can't conclude anything about the order of a reaction just by having a look at the equation for the reaction. By doing experiments involving a reaction between A and B, the rate of the reaction is identified to be related to the concentrations of A and B as follows:
This is the Rate Equation.
Where,
Rate is in the units of mol dm-3s-1
k is the rate constant
A, B- concentrations in mol dm-3
a - Order of reaction with respect to A
b- Order of reaction with respect to B
If temperature is given, the rate is usually considered to be a function of the initial concentrations of the reactants A and B.
Answer to Problem 11.35PAE
Solution: The rate law of the reaction is
Explanation of Solution
Given information: Reaction:
Experimental Data
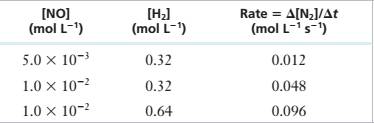
Step 1: For the reaction:
The rate law can be determined using the rate equation as follows:
Where,
a= Order of the reaction with respect to NO
b= Order of the reaction with respect to
Step 2: From the first, second and third rows of the given experimental data,
Step 3: Divide (2) by (3), we get
Step 4: Divide (1) by (2), we get
Step 5: Rate Equation = >
Step 6: Substitute a=2,b=1 values in (1)
It does not make a difference what the number of reactants there are. The concentration of every reactant will be present in the rate equation, raised to some power. These powers resemble the individual orders with respect to each reactant. The sum of these powers results in the overall order of the reaction. The rate constant will be a constant value for a given reaction only if the concentration of the reactants is changed without changing any other factors.
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Engineering Students
- Monochloroethane (C2H5Cl) can be produced by the direct reaction of ethane gas (C2H6) with chlorine gas or by the reaction of ethylene gas (C2H4) with hydrogen chloride gas. The second reaction gives almost a 100% yield of pure C2H5Cl at a rapid rate without catalysis. The first method requires light as an energy source or the reaction would not occur. Yet G for the first reaction is considerably more negative than G for the second reaction. Explain how this can be so.arrow_forward9.96 Most first aid "cold packs" are based on the endothermic dissolution of ammonium nitrate in water: NH4NO3(s)NH4+(aq)+NO3(aq) H= 25.69 kJ A particular cold pack contains 50.0 g of NH4NO3 and 125.0 g of water. When the pack is squeezed, the NH4NO3dissolves in the water. If the pack and its contents are initially at 24.0°C, what is the lowest temperature that this bag could reach? (Assume that the ammonium nitrate solution has a specific heat of 4.25J g-l K-l, and that the heat capacity of the bag itself is small enough to be neglected.)arrow_forwardThe reaction of carbon monoxide with hydrogen to form methanol is quite slow at room temperature. As a general rule, reactions go faster at higher temperatures. Suppose that you tried to speed up this reaction by increasing the temperature. (a) Assuming that rH does not change very much as the temperature changes, what effect would increasing the temperature have on rSsurroundings? (b) Assuming that rS for a reaction System does not change much as the temperature changes, what effect would increasing the temperature have on rSuniverse?arrow_forward
- Consider the reaction H2(g)+Br2(g)2HBr(g) where H = 103.8 kJ/mol. In a particular experiment, equal moles of H2(g) at 1.00 atm and Br2(g) at 1.00 atm were mixed in a 1.00-L flask at 25C and allowed to reach equilibrium. Then the molecules of H2 at equilibrium were counted using a very sensitive technique, and 1.10 1013 molecules were found. For this reaction, calculate the values of K, G, and S.arrow_forwardOld-fashioned smelling salts consist of ammonium carbonate, (NH4)2CO3. The reaction for the decomposition of ammonium carbonate (NH4)2CO3(s)2NH3(g)+CO(g)+H2O(g) is endothermic. Would the smell of ammonia increase or decrease as the temperature is increased?arrow_forwardFor the endothermic reaction AB(g)A(g)+B(g), the following represents a reaction container at two different temperatures. Which one (I or II) is at the lower temperature?arrow_forward
- Silicon forms a series of compounds analogous to the al-kanes and having the general formula SinH2n+2. The first of these compounds is silane, SiH4, which is used in the electronics industry to produce thin ultrapure silicon films. SiH4(g) is somewhat difficult to work with because it is py-ropboric at room temperature—meaning that it bursts into flame spontaneously when exposed to air. (a) Write an equation for the combustion of SiH4(g). (The reaction is analogous to hydrocarbon combustion, and SiO2 is a solid under standard conditions. Assume the water produced will be a gas.) (b) Use the data from Appendix E to calculate ? for this reaction. (c) Calculate G and show that the reaction is spontaneous at 25°C. (d) Compare G for this reaction to the combustion of methane. (See the previous problem.) Are the reactions in these two exercises enthalpy or entropy driven? Explain.arrow_forwardThe recycling of polymers represents only one industrial process that allows creating order in one location by creating greater disorder at some other location, often at a power plant. List three other industrial processes that must create disorder in the surroundings to generate the desired material.arrow_forwardConsider the reaction 2SO2(g)+O2(g)2SO3(g) (a) Calculate G at 25C. (b) If the partial pressures of SO2 and SO3 are kept at 0.400 atm, what partial pressure should O2 have so that the reaction just becomes nonspontaneous (i.e., G=+1.0 k J)?arrow_forward
- N2(g) + 3H2(g) + 2NH3(g) At 500°C, the value of Kc for this reaction is 0.40. The following concen- trations of gases are present in a container at 500°C: [N2(g)] = 0.10 mol/L, [H2(g)] = 0.30 mol/L, and [NH3(g)] = 0.20 mol/L. Is this mixture of gases at equilibrium? If not, in which direction will the reaction go to reach equilibrium? Is this mixture of gases at equilibrium: If "no", in which direction will the reaction go: (yes/no) (left/right/no-shift)arrow_forwardDraw the organic molecule(s) which is(are) formed in the following reaction. Do not include molecules like H,O or HCl. (You have 2 chances until the answer will be given; you have already tried 0 times) H, C-CH, Pt HC C-CH + H, (50 atm) heat CH,arrow_forward16) Fill the blank of the following sentences. a) When CH4(g) is removed from the reaction below, the reaction shifts to the CO(g) + 3H₂(g) = CH4(g) + H₂O(g) at equilibrium heat b) The reaction 2SO3(g) = 2SO2(g) + O2(g) is endothermic. If the temperature is reaction will shift to the left. (decreasing the c) For the following reaction at equilibrium, the equilibrium would shift to the left when temperature is (decreasing). heat 2NOBr(g) = 2NO(g) + Br₂(g), AH°rxn= -30 kJ/mol d) For the reaction of 3H₂(g) + N2(g) = 2NH3(g), if the volume of the vessel containing hydrogen and nitrogen is decreased, the production of ammonia will (decrease ). - (decrease right.arrow_forward
 General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





