The ratio of the isotopes carbon-14 (14C, radioactive and decaying) to carbon-12 (12C non-radioactive and non-decaying) is an important indicator to determine age of fossils, woods and other organic remnants of plants and animals. Assume an animal skeleton was found with a ration 14C/12C of 61% and a half-life h of 14C of 5730 years
The ratio of the isotopes carbon-14 (14C, radioactive and decaying) to carbon-12 (12C non-radioactive and non-decaying) is an important indicator to determine age of fossils, woods and other organic remnants of plants and animals. Assume an animal skeleton was found with a ration 14C/12C of 61% and a half-life h of 14C of 5730 years
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter25: Nuclear Chemistry
Section25.4: Rates Of Nuclear Decay
Problem 25.5CYU
Related questions
Question
The ratio of the isotopes carbon-14 (14C, radioactive and decaying) to carbon-12 (12C non-radioactive and non-decaying) is an important indicator to determine age of fossils, woods and other organic remnants of plants and animals. Assume an animal skeleton was found with a ration 14C/12C of 61% and a half-life h of 14C of 5730 years.
How long ago did the animal die?
State your result as a whole number including the units.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
When we were given the attached formula, how can I get the answer?
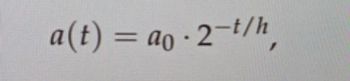
Transcribed Image Text:a(t)
-ao-2-t/h
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning