tab Worksheet-Polarity of Bonds I. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form between atoms of the following elements and show the polarity of the bond if it is polar covalent. a. Ca and Cl ionic 3.0-1.0 2.0 b. C and S non-polar 2.5-2.5= O c. Mg and Fionic d. N and O polar 3.5-3.0= 0.5 e. H and O polar 3.5-2.1=1.4 f. S and O polar 4.0-1.2=38 3.5-2.5: 1.0 II. The bonds between the following pairs of elements are covalent. Arrange them according to polarity, naming the most polar bond first on the side of the sheet. shif a. H-CI 3.0-2.1=0.9 b. H-C 2.5-2.1=0.4 ctr c. H-F 4.0-2.1=1.9 d. H-0 3.5-2.1=1.4 e. H-H 2-1-2.1=0 f. S-Cl 3.0-2.5= 0.5 III. Using the table of electronegativities from your Periodic table, calculate the EN difference for the atoms that are bonded in the following molecules. Then tell whether the bond is nonpolar covalent, polar covalent, or ionic. Tell which atom has the greater share of the bonding electrons. In your drawing, show which atom is partially positive or partially negative if it is a polar covalent bond. Molecule PC13 EN Difference (between the the central atom and the outer ones) 3.0.2.1 0.9 Type of Bond polar Atom with greater EN Lewis Structure [ ? CI NH3 30-2.1 CICI 0.9 Polar N H H₂O 1.4 3.5-1.0 CaO 2.5 Ionic 3.5=2.1 H polar 0 I Ca followed. A er understand that Pent or methods of watching the participate. Finally, I/we understand that there at the school system will provide proper supervi activity if proper procedures for viewing a Kno + || = tab Worksheet-Polarity of Bonds I. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form between atoms of the following elements and show the polarity of the bond if it is polar covalent. a. Ca and Cl ionic 3.0-1.0 2.0 b. C and S non-polar 2.5-2.5= O c. Mg and Fionic d. N and O polar 3.5-3.0= 0.5 e. H and O polar 3.5-2.1=1.4 f. S and O polar 4.0-1.2=38 3.5-2.5: 1.0 II. The bonds between the following pairs of elements are covalent. Arrange them according to polarity, naming the most polar bond first on the side of the sheet. shif a. H-CI 3.0-2.1=0.9 b. H-C 2.5-2.1=0.4 ctr c. H-F 4.0-2.1=1.9 d. H-0 3.5-2.1=1.4 e. H-H 2-1-2.1=0 f. S-Cl 3.0-2.5= 0.5 III. Using the table of electronegativities from your Periodic table, calculate the EN difference for the atoms that are bonded in the following molecules. Then tell whether the bond is nonpolar covalent, polar covalent, or ionic. Tell which atom has the greater share of the bonding electrons. In your drawing, show which atom is partially positive or partially negative if it is a polar covalent bond. Molecule PC13 EN Difference (between the the central atom and the outer ones) 3.0.2.1 0.9 Type of Bond polar Atom with greater EN Lewis Structure [ ? CI NH3 30-2.1 CICI 0.9 Polar N H H₂O 1.4 3.5-1.0 CaO 2.5 Ionic 3.5=2.1 H polar 0 I Ca followed. A er understand that Pent or methods of watching the participate. Finally, I/we understand that there at the school system will provide proper supervi activity if proper procedures for viewing a Kno + || =
tab Worksheet-Polarity of Bonds I. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form between atoms of the following elements and show the polarity of the bond if it is polar covalent. a. Ca and Cl ionic 3.0-1.0 2.0 b. C and S non-polar 2.5-2.5= O c. Mg and Fionic d. N and O polar 3.5-3.0= 0.5 e. H and O polar 3.5-2.1=1.4 f. S and O polar 4.0-1.2=38 3.5-2.5: 1.0 II. The bonds between the following pairs of elements are covalent. Arrange them according to polarity, naming the most polar bond first on the side of the sheet. shif a. H-CI 3.0-2.1=0.9 b. H-C 2.5-2.1=0.4 ctr c. H-F 4.0-2.1=1.9 d. H-0 3.5-2.1=1.4 e. H-H 2-1-2.1=0 f. S-Cl 3.0-2.5= 0.5 III. Using the table of electronegativities from your Periodic table, calculate the EN difference for the atoms that are bonded in the following molecules. Then tell whether the bond is nonpolar covalent, polar covalent, or ionic. Tell which atom has the greater share of the bonding electrons. In your drawing, show which atom is partially positive or partially negative if it is a polar covalent bond. Molecule PC13 EN Difference (between the the central atom and the outer ones) 3.0.2.1 0.9 Type of Bond polar Atom with greater EN Lewis Structure [ ? CI NH3 30-2.1 CICI 0.9 Polar N H H₂O 1.4 3.5-1.0 CaO 2.5 Ionic 3.5=2.1 H polar 0 I Ca followed. A er understand that Pent or methods of watching the participate. Finally, I/we understand that there at the school system will provide proper supervi activity if proper procedures for viewing a Kno + || = tab Worksheet-Polarity of Bonds I. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form between atoms of the following elements and show the polarity of the bond if it is polar covalent. a. Ca and Cl ionic 3.0-1.0 2.0 b. C and S non-polar 2.5-2.5= O c. Mg and Fionic d. N and O polar 3.5-3.0= 0.5 e. H and O polar 3.5-2.1=1.4 f. S and O polar 4.0-1.2=38 3.5-2.5: 1.0 II. The bonds between the following pairs of elements are covalent. Arrange them according to polarity, naming the most polar bond first on the side of the sheet. shif a. H-CI 3.0-2.1=0.9 b. H-C 2.5-2.1=0.4 ctr c. H-F 4.0-2.1=1.9 d. H-0 3.5-2.1=1.4 e. H-H 2-1-2.1=0 f. S-Cl 3.0-2.5= 0.5 III. Using the table of electronegativities from your Periodic table, calculate the EN difference for the atoms that are bonded in the following molecules. Then tell whether the bond is nonpolar covalent, polar covalent, or ionic. Tell which atom has the greater share of the bonding electrons. In your drawing, show which atom is partially positive or partially negative if it is a polar covalent bond. Molecule PC13 EN Difference (between the the central atom and the outer ones) 3.0.2.1 0.9 Type of Bond polar Atom with greater EN Lewis Structure [ ? CI NH3 30-2.1 CICI 0.9 Polar N H H₂O 1.4 3.5-1.0 CaO 2.5 Ionic 3.5=2.1 H polar 0 I Ca followed. A er understand that Pent or methods of watching the participate. Finally, I/we understand that there at the school system will provide proper supervi activity if proper procedures for viewing a Kno + || =
ChapterU2: Smells: Molecular Structure And Properties
Section: Chapter Questions
Problem 3STP
Related questions
Question
Transcribed Image Text:tab
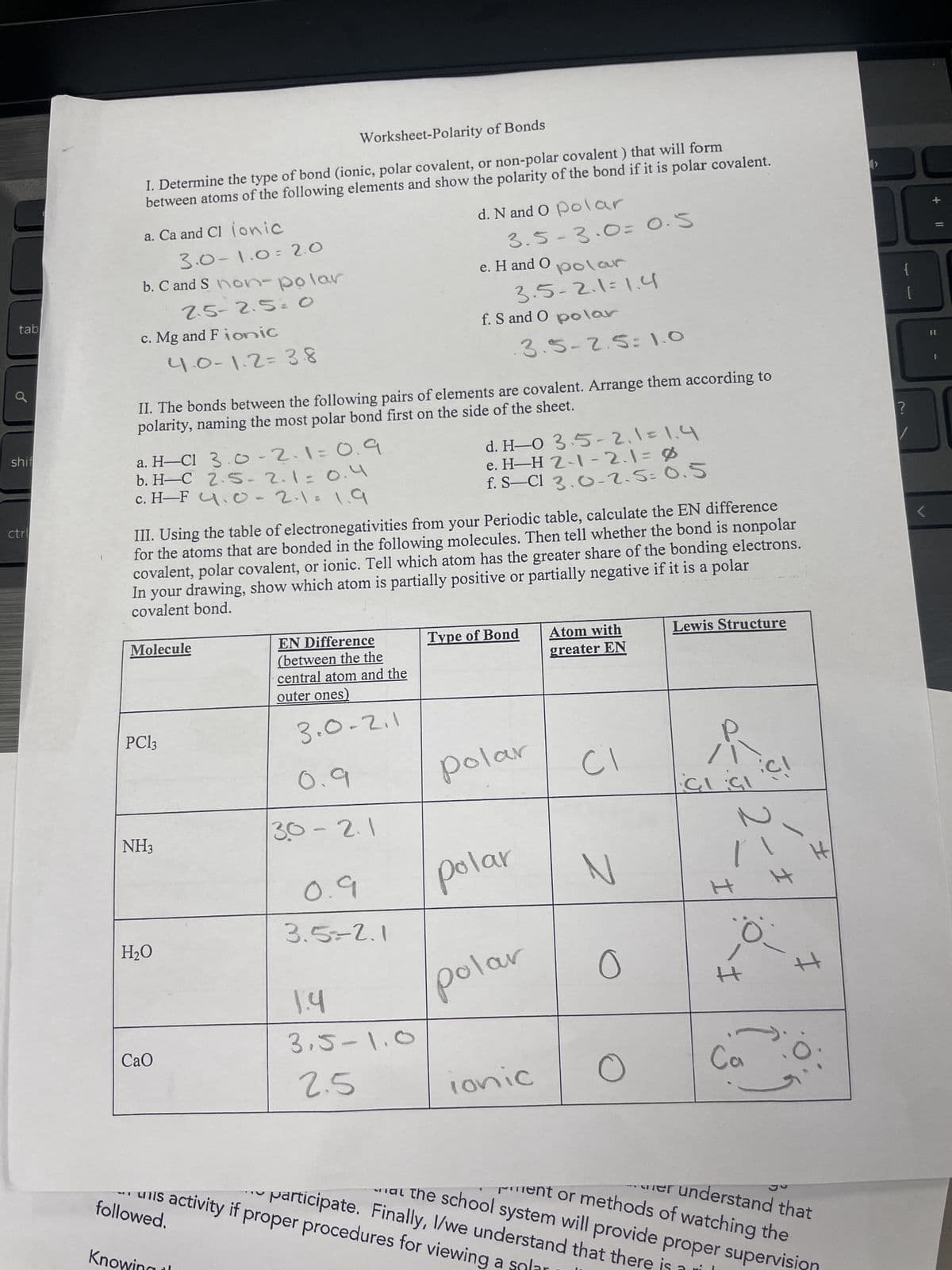
Worksheet-Polarity of Bonds
I. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form
between atoms of the following elements and show the polarity of the bond if it is polar covalent.
a. Ca and Cl ionic
3.0-1.0 2.0
b. C and S non-polar
2.5-2.5= O
c. Mg and Fionic
d. N and O polar
3.5-3.0= 0.5
e. H and O
polar
3.5-2.1=1.4
f. S and O polar
4.0-1.2=38
3.5-2.5: 1.0
II. The bonds between the following pairs of elements are covalent. Arrange them according to
polarity, naming the most polar bond first on the side of the sheet.
shif
a. H-CI 3.0-2.1=0.9
b. H-C 2.5-2.1=0.4
ctr
c. H-F 4.0-2.1=1.9
d. H-0 3.5-2.1=1.4
e. H-H 2-1-2.1=0
f. S-Cl 3.0-2.5= 0.5
III. Using the table of electronegativities from your Periodic table, calculate the EN difference
for the atoms that are bonded in the following molecules. Then tell whether the bond is nonpolar
covalent, polar covalent, or ionic. Tell which atom has the greater share of the bonding electrons.
In your drawing, show which atom is partially positive or partially negative if it is a polar
covalent bond.
Molecule
PC13
EN Difference
(between the the
central atom and the
outer ones)
3.0.2.1
0.9
Type of Bond
polar
Atom with
greater EN
Lewis Structure
[
?
CI
NH3
30-2.1
CICI
0.9
Polar
N
H
H₂O
1.4
3.5-1.0
CaO
2.5
Ionic
3.5=2.1
H
polar
0
I
Ca
followed.
A
er understand that
Pent or methods of watching the
participate. Finally, I/we understand that there
at the school system will provide proper supervi
activity if proper procedures for viewing a
Kno
+ ||
=
Transcribed Image Text:tab
Worksheet-Polarity of Bonds
I. Determine the type of bond (ionic, polar covalent, or non-polar covalent) that will form
between atoms of the following elements and show the polarity of the bond if it is polar covalent.
a. Ca and Cl ionic
3.0-1.0 2.0
b. C and S non-polar
2.5-2.5= O
c. Mg and Fionic
d. N and O polar
3.5-3.0= 0.5
e. H and O
polar
3.5-2.1=1.4
f. S and O polar
4.0-1.2=38
3.5-2.5: 1.0
II. The bonds between the following pairs of elements are covalent. Arrange them according to
polarity, naming the most polar bond first on the side of the sheet.
shif
a. H-CI 3.0-2.1=0.9
b. H-C 2.5-2.1=0.4
ctr
c. H-F 4.0-2.1=1.9
d. H-0 3.5-2.1=1.4
e. H-H 2-1-2.1=0
f. S-Cl 3.0-2.5= 0.5
III. Using the table of electronegativities from your Periodic table, calculate the EN difference
for the atoms that are bonded in the following molecules. Then tell whether the bond is nonpolar
covalent, polar covalent, or ionic. Tell which atom has the greater share of the bonding electrons.
In your drawing, show which atom is partially positive or partially negative if it is a polar
covalent bond.
Molecule
PC13
EN Difference
(between the the
central atom and the
outer ones)
3.0.2.1
0.9
Type of Bond
polar
Atom with
greater EN
Lewis Structure
[
?
CI
NH3
30-2.1
CICI
0.9
Polar
N
H
H₂O
1.4
3.5-1.0
CaO
2.5
Ionic
3.5=2.1
H
polar
0
I
Ca
followed.
A
er understand that
Pent or methods of watching the
participate. Finally, I/we understand that there
at the school system will provide proper supervi
activity if proper procedures for viewing a
Kno
+ ||
=
AI-Generated Solution
Unlock instant AI solutions
Tap the button
to generate a solution
Recommended textbooks for you


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
