
Concept explainers
Three resonance structures are possible for dinitrogen monoxide, N2O.
- (a) Draw the three resonance structures.
- (b) Calculate the formal charge on each atom in each resonance structure.
- (c) Based on formal charges and electronegativity, predict which resonance structure is the most reasonable.
(a)
Interpretation:
The three resonance structure of
Concept Introduction:
Resonance structures: A molecule or ion which show more than structure but none of them are accurately correct show the known property of that molecule, and can lie between the canonical structure is known as resonance or canonical or contributing structure.
Explanation of Solution
The three resonance structure is drawn
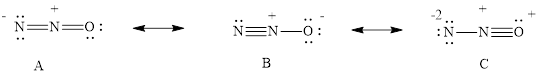
(b)
Interpretation:
Formal charge on each atom in each resonance structure has to be calculated.
Concept Introduction:
Formal charge: It is the electrostatic charge that would reside on an atom in a molecule or polyatomic ion if all bonding electron are shared equally between pairs of atoms.
Formal charge calculation: The formal charge for atom in a molecule or ion is calculated based on the Lewis structure of the molecule or ion by following the given equation below:

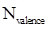 - Number of valence electrons
- Number of valence electrons
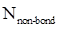 - Number of non-bonding electrons
- Number of non-bonding electrons
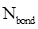 - Number of bonding electrons
- Number of bonding electrons
Explanation of Solution
The formal charges can be calculated as follows.
For resonance structure A is given below,
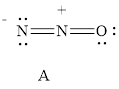
Formal charge on nitrogen
Formal charge on nitrogen
Formal charge on oxygen can be calculated as follows.
For resonance structure B is given below,
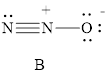
Formal charge on nitrogen
Formal charge on nitrogen
Formal charge on oxygen can be calculated as follows.
For resonance structure C, is given below,
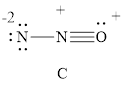
Formal charge on nitrogen
Formal charge on nitrogen
Formal charge on oxygen can be calculated as follows.
(c)
Interpretation:
From the resonance structure drawn, the most reasonable structure has to be identified.
Concept Introduction:
Formal charge: It is the electrostatic charge that would reside on an atom in a molecule or polyatomic ion if all bonding electron are shared equally between pairs of atoms.
Formal charge calculation: The formal charge for atom in a molecule or ion is calculated based on the Lewis structure of the molecule or ion by following the given equation below:

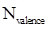 - Number of valence electrons
- Number of valence electrons
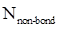 - Number of non-bonding electrons
- Number of non-bonding electrons
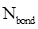 - Number of bonding electrons
- Number of bonding electrons
Resonance structures:
A molecule or ion which show more than structure but none of them are accurately correct show the known property of that molecule, and can lie between the canonical structure is known as resonance or canonical or contributing structure.
Explanation of Solution
The three resonance structure is drawn
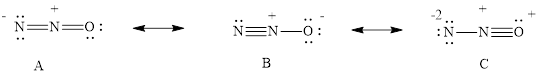
- (a) The formal charges can be calculated as follows.
For resonance structure A
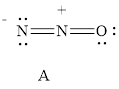
Formal charge on nitrogen
Formal charge on nitrogen
Formal charge on oxygen can be calculated as follows.
For resonance structure B
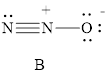
Formal charge on nitrogen
Formal charge on nitrogen
Formal charge on oxygen can be calculated as follows.
For resonance structure C
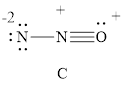
Formal charge on nitrogen
Formal charge on nitrogen
Formal charge on oxygen can be calculated as follows.
Thus from the formal charge given above, the Structure B is most reasonable
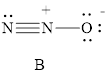
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry & Chemical Reactivity
- (a) Describe the molecule xenon trioxide, XeO3, using four possible Lewis structures, one each with zero, one, two, or three Xe—O double bonds. (b) Do any of these resonance structures satisfy the octet rule for every atom in the molecule? (c) Do any of the four Lewis structures have multiple resonance structures? If so, how many resonance structures do you find? (d) Which of the Lewis structures in (a) yields the most favorable formal charges for the molecule?arrow_forwardConsider the formate ion, HCO2", which is the anion formed when formic acid loses an H* ion. The H and the two O atoms are bonded to the central C atom. (a) Draw the best Lewis structure(s) for this ion. (b) Are resonance structures needed to describe the structure? Explain briefly (c) Would you predict that the C-O bond lengths in the formate ion would be longer or shorter relative to those in CO2? Explain brieflyarrow_forwardThe two compounds nitrogen dioxide and dinitrogentetraoxide are introduced in Section 3.13.(a) NO2 is an odd-electron compound. Draw the bestLewis diagrams possible for it, recognizing that oneatom cannot achieve an octet configuration. Use formal charges to decide whether that should be the(central) nitrogen atom or one of the oxygen atoms.(b) Draw resonance forms for N2O4 that obey the octetrule. The two N atoms are bonded in this molecule.arrow_forward
- A stable triatomic molecule can be formed that contains one atom each of nitrogen, sulfur, and fluorine. Three bonding structures are possible, depending on which is the central atom: NSF, SNF, and SFN. (a) Write a Lewis diagram for each of these molecules, indicating the formal charge on each atom. (b) Often, the structure with the least separation of formal charge is the most stable. Is this statement consistent with the observed structure for this molecule— namely, NSF, which has a central sulfur atom? (c) Does consideration of the electronegativities of N, S, and F from Figure 3.18 help rationalize this observed structure? Explain. 100. The gasarrow_forward(a) Determine the formal charge of oxygen in the following structure. If the atom is formally neutral, indicate a charge of zero. (b) Draw an alternative Lewis (resonance) structure for the compound given in part (a). Show the unshared pairs and nonzero formal charges in your structure. Don't use radicals. Formal charge on O 0arrow_forward3) The molecule diphosphorus tetraoxide (P,O,) has two central atoms and four different resonance structures that do not violate the octet rule. Draw two of these resonance structures below. 4) The compound acetone is a common solvent. It has a chemical formula of CH,COCH, Acetone has three central atoms. (a) Draw the Lewis Dot structure for acetone. (b) Give the Ideal Bond Angle for all three central atoms. 5) Four covalent molecules are drawn below. :o: H. H-CH H H (1) (2) (3) (4) a) Define each of these molecules as polar or non-polar. (1) (2) (3) b) Describe the type of intermolecular force that each molecule would use: (1) (2) (3) (4)arrow_forward
- Draw Lewis structures for HFO4, HFO3, HC0O4, HC0O3, HCO2. (These molecules have the halogen atom as the central atom. All O atoms are attached to the halogen. The hydrogen atom is bonded to one of the O atoms.) Use formal charges to determine which molecule is least likely to occur in nature. (A) HFO4 (B) HC(O2 (C) HC!O3 (D) HFO3 (E) HC\O4 DO000arrow_forwardThree resonance structures are possible for the thiocyanate ion, SCN-. (a) Draw the three resonance structures. (b) Calculate the formal charge on each atom in each resonance structures. (c) Based on formal charges and electronegativity, predict which resonance structure most closely approximates the bonding in this ion? (d) What are the similarities and differences of bonding in SCN compared to the bonding in OCN- .arrow_forwardCalculate the enthalpy change for the following reactions using the bond enthalpy given below. (Bond enthalpy/kJ : H−H = 436, C−H = 413, C=O = 799, O=O = 495, O−H = 463) (a) H2(g) + 1⁄2O2(g) → H2O(g) (b) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)arrow_forward
- Consider the collection of nonmetallic elements O, P, Te,I, and B. (a) Which two would form the most polar singlebond? (b) Which two would form the longest single bond?(c) Which two would be likely to form a compound of formulaXY2? (d) Which combinations of elements would likelyyield a compound of empirical formula X2Y3?arrow_forwardWhich of these statements about resonance is true?(a) When you draw resonance structures, it is permissibleto alter the way atoms are connected.(b) The nitrate ion has one long N¬O bond and two shortN¬O bonds.(c) “Resonance” refers to the idea that molecules areresonating rapidly between different bonding patterns.(d) The cyanide ion has only one dominant resonancestructure.(e) All of the above are true.arrow_forwardIn the vapor phase, BeCl2 exists as a discrete molecule. (a) Draw the Lewis structure of this molecule, using only single bonds. Does this Lewis structure satisfy the octet rule? (b) What other resonance structures are possible that satisfy the octet rule? (c) On the basis of the formal charges, which Lewis structure is expected to be dominant for BeCl2?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
