
Organic Chemistry: A Guided Inquiry
2nd Edition
ISBN: 9780618974122
Author: Andrei Straumanis
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Please show it drawn out.
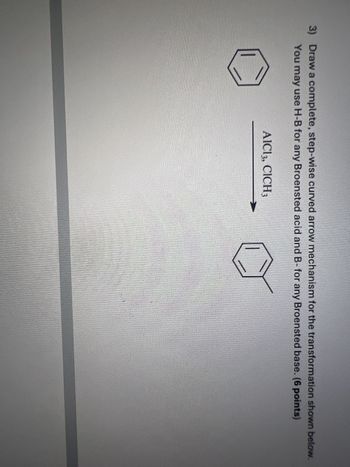
Transcribed Image Text:3) Draw a complete, step-wise curved arrow mechanism for the transformation shown below.
You may use H-B for any Broensted acid and B- for any Broensted base. (6 points)
AlCl3, CICH 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Using ChemDraw Draw a mechanism of a reversible reaction of acetophenone and strong acid (H-A) to form a protonated acetophenone and a weak base. Draw all the electron pairs of the molecules and the arrows.arrow_forward1. (10 points). Draw a stepwise detailed mechanism for the following reaction showing all the substitution and the elimination products formed. Label the type of the mechanism (SN1, SN2, E1 or E2). Show stereochemistry when applicable. Br CH3CH2OH H₂O 2. (10 points) 0 Br CH3O Na DMSO Draw the detailed mechanism showing ALL possible products formed including stereochemistry and Label mechanism as SN1, SN2, E1 or E2. Show stereochemistry when applicable.arrow_forward3. (10 points) Complete the reaction scheme below. Show all reagents and intermediates. No reaction is a possible answer. (C6H5)3P= C- o-C2H5arrow_forward
- (5 points) Identify the electrophile and the nucleophile in each of the following reaction steps and then draw curved arrows to illustrate the bond-making and bond-breaking processes. C'H;CH CH, + Н-Br CНCH -СH + Brarrow_forward4. (10 points) Complete the reaction scheme below. Show all reagents and intermediates. No reaction is a possible answer. LDA lodomethane CH3 H Harrow_forward2) a) Draw the product if Molecule A underwent a [3,3]-sigmatropic rearrangement. Hint, you may want to start by redrawing the starting materials in their reactive conformations. (4 points) Molecule A CH3 CH2 b) Show the complete, step-wise curved arrow mechanism for the above reaction. You may use H-B for any Broensted acid and B- for any Broensted base. You can maximize your chances of partial credit by providing a consistent numbering scheme for your carbons. (9 points)arrow_forward
- Please Write SteP by Step Answer Otherwise I give you DISLIKES !arrow_forwardMechanism: Draw a stepwise mechanism for the following reaction. Show all intermediate structures and all electron flow using curved arrows. Label the kinetic and thermodynamic product. Your mechanism must include all intermediate structures and all electron flow with curved arrows. You must include all formal charges and lone pairs of electrons for full credit! Br H-Br Br +arrow_forward1. Please name the following compounds. (9 points) Br CI Br Br 2. Please draw the major product(s) of the following reaction and show the mechanism. Is there a reason why one stereochemistry is preferred over the other for this reaction? (9 points)arrow_forward
- 6. (6 points) Suggest an efficient synthesis for the following transformation. Draw the major product at each step and indicate all reagents used. Br میں مملarrow_forwardDehydration of 1,2,2-trimethylcyclohexanol with H2SO4 affords 1-tertbutylcyclopentene as a minor product. (a) Draw a stepwise mechanism that shows how this alkene is formed. (b) Draw other alkenes formed in this dehydration. At least one must contain a five-membered ring.arrow_forwardSynthesis: Show allof the reagents needed to transform the starting material on the left to the product on the right. You do NOT need to show the mechanisms for the reactions. (9 points) он "OH 18.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning