What is the meaning of solidification?
The term solidification indicates the conversion of material from the state of liquid to the state of solid. The solid-state is more compact and rigid than liquid, and the solidification indicates the transformation of material from a random state to a more compact and crystalline form. The point at which dendrite formation in liquid starts taking place is referred to as the solidification point.
What is the main cause of solidification?
The energy of the liquid is less than that of the solid above the melting point. Hence the liquid is stable above its melting point. The energy of liquid becomes more than the solid below the melting point temperature. So, below the melting point, the solid becomes more stable than the liquid. At the melting point, liquid gets converted into solid during cooling.

Solidification point
Whenever a material is in liquid state at a specific temperature and pressure, the temperature starts decreasing up to a point called the solidification point. This generates a temperature gradient. Due to this temperature gradient, material solidification from the liquid to the solid state starts taking place.
Process of solidification of metals
Pure metals have a constant melting temperature, just like other elements in nature. For pure metals, the melting point and freezing point are also equal to each other. Whenever a specific liquid metal at a temperature higher than its melting point is poured into a mold cavity, the solidification process starts after some time from the mold wall. The complete solidification of liquid metal is categorized into three different processes, which are given below:
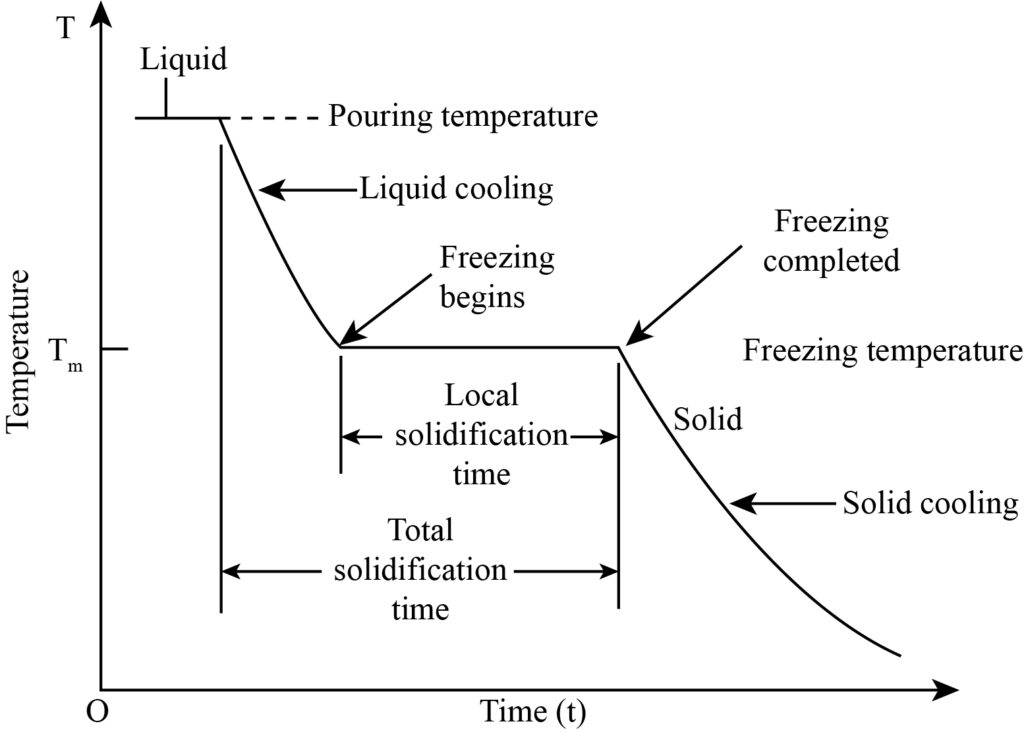
- When the liquid metal is poured into the mold cavity with a higher temperature than the melting point, cooling starts taking place up to the freezing point temperature that is referred to as liquid cooling.
- After the cooling of the liquid, the liquid to solid transformation starts taking place at a constant temperature in a specific time period known as the phase transformation process.
- At last, when the freezing of liquid is complete, solid cooling starts taking place at a particular time to room temperature.
Stages of solidification of metals
The solidification process consists of successive stages of nucleation and growth. In the first stage, at the surface of the mold wall, nucleation starts. After the nucleation, the growth of nuclei takes place with respect to time.
Nucleation
When a liquid metal is poured into a mold cavity, liquid metal makes contact with the wall of the mold. The process of cooling of the liquid metal starts near the wall surface. Thus the process of nucleation starts at the wall surface. The size of nucleation is very tiny in form of small droplets.
Dendrites growth
After the nucleation, when the solidification proceeds into the inner part of the mold, the grains would convert into spikes positioned inside the mold. Until the full solidification of pure metals, the spike grains would convert into big branches that are positioned inside the mold. These big branches of molecular structures unite in the innermost section of the mold cavity. This solidification phenomenon of metals is called dendrite growth.

Types of nucleation
The process of nucleation can take place in two different ways which are homogeneous nucleation and heterogeneous nucleation. The short details about these two types of nucleation are given below in the following steps:
Homogeneous nucleation
It is a type of nucleation in which nucleation occurs due to the random motion of liquid atoms away from the system/wall of the mold cavity occurs. In the homogeneous nucleation, the ordered bunch of atoms forms a high-density zone.
Heterogeneous nucleation
It is a type of nucleation in which nucleation due to the random motion of liquid atoms starts at the system/wall of the mold cavity. For heterogeneous nucleation, the initial interface for growth is provided by a foreign particle. This foreign particle may be available from outside or formed in the liquid metal itself. The impurities, foreign particles, or even the mold wall (the substrate) can provide part of the surface energy required for nucleation.

Free energy change during nucleation
Whenever the nucleation during the solidification of metal takes place, there is a change in free energy. The total free energy change consists of volume free energy change and interfaces free energy change.
Volume free energy change
Whenever a solid comes out as a liquid, then there is a change of negative free energy that takes place in the system. The value of this change in free energy is directly proportional to the new volume of solid forms.
Interface free energy change
Whenever a new interface forms due to a generation of solid from liquid, then there would be an increase in the free energy. This increment in the free energy takes place at the new interface that forms, which is referred to as the interface free energy change. The value of this free energy change is directly proportional to the surface area of the interface forms.
The expression of the inter-facial free energy change can be represented as,
Here, represents the interface free energy change, represents the radius of new solid and represents the inter-facial free energy per unit area.
Plane front growth
The nucleation during solidification of a liquid metal does not take place randomly; it takes place uniformly at the surface of the mold wall, and the growth of the dendrites that form after nucleation is directed towards the center of mold uniformly. The growth of the solid phase starts from the plane surface of the mold and moves uniformly towards the mold's center.

Factors affecting solidification of metals
There are various factors that affect the solidification of metals. Some of the factors increase the solidification rate, whereas some of the factors decrease the solidification rate of metals. The factors affecting the solidification of the metals are as follows:
- Cooling Rate
- Foreign atoms/impurities
- Alloy constituents
- Thermal conductivity
- Degree of superheat
The details about the factors are given below:
Cooling rate
When a liquid metal starts to solidify to a solid phase, the cooling rate plays an important role. If the cooling rate is higher, it means the rate of heat energy loss is higher, resulting in the formation of a fine grain structure of solid. If the cooling rate is slow like furnace cooling, then there coarse grain structure forms during solidification.
Foreign atom/impurities
Whenever a foreign atom or impurity is present in liquid metal, then there would be heterogeneous nucleation preferred because heterogeneous nucleation starts taking place at the surface of a foreign atom.
Alloy constituents
Different materials have different constituents, and the solidification of different materials depends on the constituents of the material. If the material is crystalline in nature, then the microstructure during solidification is different from a non-crystalline material. The solidification patterns of metal are different from non-metal.
Thermal conductivity
Whenever a liquid metal is poured into a mold cavity, and the thermal conductivity of mold is higher, the heat transfer rate would be more from the mold cavity to the environment. It results in faster cooling, and the formation of fine grain structure takes place.
Degree of superheat
The term degree of superheat represents the extra temperature above that of the melting temperature of the metal. If the degree of superheat is more, the temperature gradient would be more and result in faster cooling. Due to this, the formation of fine grain structure takes place.
Common Mistakes
- Students sometimes get confused regarding the difference between homogeneous and heterogeneous nucleation. However, homogeneous nucleation takes place away from the mold wall, whereas heterogeneous nucleation starts at the surface of the mold wall.
- Sometimes, students also get confused about why material is more stable in solid form in comparison to liquid or gaseous forms. However, during the conversion of liquid metal to its solid-state, there would be a decrement in energy. So, the material would be stable at the lowest energy level means in the solid-state.
- The student also gets confused about when fine grain structure and coarse grain, structures are obtained in the solidification of the metal. However, if the cooling rate is fast, then a fine grain structure is obtained, and if the cooling rate is less, then the coarse grain structure is obtained.
Context and Applications
Solidification of metals is very significant in the several professional exams and courses for undergraduate, Diploma level, graduate, postgraduate. For example:
- Bachelor of Technology in Mechanical Engineering
- Bachelor of Technology in Metallurgical and Materials Engineering
- Master of Technology in Mechanical and Metallurgical and Materials Engineering
- Doctor of Philosophy in Mechanical Engineering
Related Concepts
- Dendrites formation
- Cooling curve
- Free energy
- Shrinkage of metals
- Shrinkage defects
- Solidification time
- Solidification imperfections
Practice Problems
Q1. When a metal solidifies in the presence of slow cooling, what types of grain form?
a. Columnar grains
b. Fine grains
c. Equiaxed grains
d. None of these
Correct option: (a)
Explanation: The size of grains formed in the material is based on the type or rate of cooling. The columnar grains are formed in metals during the slow cooling process. In contrast, faster cooling rates produce finer grain structures.
Q2. When a metal solidifies in the presence of a fast cooling rate, what types of grain form?
a. Columnar grains
b. Fine grains
c. Equiaxed grains
d. None of these
Correct option: (b)
Explanation: The faster cooling rate produces fine grain structures, while slow cooling produces coarse grain structures.
Q3. What is the manner of crystal growth during the solidification of a metal?
a. Granular
b. Linear
c. Dendritic
d. None of these
Correct option: (c)
Explanation: When the solidification proceeds into the inner part of the mold, the grains would convert into spikes positioned inside the mold, then these small spikes combine and form big branches of molecular structures in the innermost section of the mold cavity. This solidification phenomenon of metals is called dendrite growth.
Q4. Which of the following point, the material solidification from the liquid to the solid-state starts taking place?
a. Freezing point
b. Boiling point
c. Solidification point
d. Cryogenic point
Correct option: (c)
Explanation: The material solidification from the liquid to the solid-state starts at the solidification point or solidification temperature. Its value is different for different materials.
Q5. Most of the metal during solidification gets
a. Shrinkage
b. Expand
c. Elongate
d. No change
Correct option: (a)
Explanation: Generally, most of the metals expand on heating and shrink on cooling.
Want more help with your mechanical engineering homework?
*Response times may vary by subject and question complexity. Median response time is 34 minutes for paid subscribers and may be longer for promotional offers.
Search. Solve. Succeed!
Study smarter access to millions of step-by step textbook solutions, our Q&A library, and AI powered Math Solver. Plus, you get 30 questions to ask an expert each month.
Materials Science and Engineering
Solidification and Crystalline Imperfections
Solidification of Metals
Solidification of metals Homework Questions from Fellow Students
Browse our recently answered Solidification of metals homework questions.
Search. Solve. Succeed!
Study smarter access to millions of step-by step textbook solutions, our Q&A library, and AI powered Math Solver. Plus, you get 30 questions to ask an expert each month.