
Organic Chemistry
8th Edition
ISBN: 9781337516402
Author: Brown
Publisher: Cengage
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8.5, Problem 8.4P
Interpretation Introduction
Interpretation:
A pair of chain propagation steps for the radical bromination of propane to give 1-bromopropane has to be written and
Concept introduction:
Halogenation of
Radical chain reaction:
Initiation reaction: 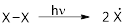
Chain propagation: 
Chain termination:
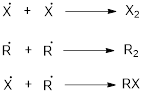
It is a change in enthalpy of a homolysis reaction at absolute zero where a molecule is broken down into two free radicals.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Define the Mechanism of the Radical Addition of HBr to an Alkene ?
Write the propagation steps leading to the formation of dichloromethane (CH2Cl2) from chloromethane
Peroxides are often added to free-radical reactions as initiators because the oxygen–oxygen bond cleaves homolytically rather easily. For example, the bond-dissociation enthalpy of the O¬O bond in hydrogen peroxide (H¬O¬O¬H) is only 213 kJ>mol (51 kcal>mol). Give a mechanism for the hydrogen peroxide-initiated reaction of cyclopentane with chlorine. The BDE for HO¬Cl is 210 kJ>mol (50 kcal>mol).
Chapter 8 Solutions
Organic Chemistry
Ch. 8.2 - Prob. 8.1PCh. 8.4 - Name and draw structural formulas for all...Ch. 8.4 - Using the table of bond dissociation enthalpies in...Ch. 8.5 - Prob. 8.4PCh. 8.6 - Given the solution to Example 8.5, predict the...Ch. 8.7 - Prob. 8.6PCh. 8.7 - Linoleic acid is shown below. What makes this...Ch. 8.7 - Prob. BQCh. 8.7 - Prob. CQCh. 8.7 - The strength of the HO bond in vitamin E is weaker...
Ch. 8.7 - Prob. EQCh. 8.8 - Prob. 8.7PCh. 8 - Prob. 8.8PCh. 8 - Prob. 8.9PCh. 8 - Prob. 8.10PCh. 8 - Prob. 8.11PCh. 8 - Account for the fact that among the chlorinated...Ch. 8 - Name and draw structural formulas for all possible...Ch. 8 - Prob. 8.14PCh. 8 - There are three constitutional isomers with the...Ch. 8 - Following is a balanced equation for bromination...Ch. 8 - Prob. 8.17PCh. 8 - Prob. 8.18PCh. 8 - Prob. 8.19PCh. 8 - Cyclobutane reacts with bromine to give...Ch. 8 - Prob. 8.21PCh. 8 - Following is a balanced equation for the allylic...Ch. 8 - Prob. 8.23PCh. 8 - Prob. 8.24PCh. 8 - The major product formed when methylenecyclohexane...Ch. 8 - Prob. 8.26PCh. 8 - Prob. 8.27PCh. 8 - Prob. 8.28PCh. 8 - Write the products of the following sequences of...Ch. 8 - Using your reaction roadmap as a guide, show...Ch. 8 - Prob. 8.31PCh. 8 - Prob. 8.32PCh. 8 - Prob. 8.33PCh. 8 - Prob. 8.34P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The compound below is treated with chlorine in the presence of light. CH3 CH3CHCH,CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. You do not have to explicitly draw H atoms.arrow_forwardThe compound below is treated with chlorine in the presence of light. CH3CH2CH2CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond.arrow_forwardFree‑radical halogenation can occur with chlorine and a source of direct UV radiation or sunlight. Chlorination of 2,4‑dimethylpentane via radical halogenation leads to the formation of all three of the products shown. Estimate the relative percentages of each product that will be formed using this means of halogenation. Presume that 1 equivalent of chlorine is used.arrow_forward
- The compound below is treated with chlorine in the presence of light. CH3 CH3CHCH₂CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. n [ ]# ?arrow_forwardThe rate law for addition of Br2 to an alkene is first orderin Br2 and first order in the alkene. Does this informationsuggest that the mechanism of addition of Br2 to analkene proceeds in the same manner as for addition of HBr?Explain.arrow_forwardThe compound below is treated with chlorine in the presence of light. CH3 CH3 CH3 Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond. • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. Sn [F ?arrow_forward
- A benzene ring alters the reactivity of a neighboring group in the so-called “benzylic” position, similarly to how a double bond alters the reactivity of groups in the “allylic” position. Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates. a) Use resonance structures to show the delocalization of the positive charge, negative charge, and unpaired electron of the benzyl cation, anion, and radical.arrow_forwardExplain why the bond dissociation enthalpy of a C-H bond in benzene is significantly greater than that in alkanesarrow_forwardThe compound below is treated with chlorine in the presence of light. CH3 CH3 [References] CH₂ Draw the structure for the organic radical species produced by reaction of the compound with a chlorine atom. Assume reaction occurs at the weakest C-H bond.arrow_forward
- The relative rates of reaction of ethane, toluene, and ethylbenzene with bromine atoms have been measured. The most reactive hydrocarbon undergoes hydrogen atom abstraction a million times faster than does the least reactive one. Arrange these hydrocarbons order of decreasing reactivity.arrow_forwardTwo substitution products result from the reaction between 3-chloro-3-methyl-1- butene with sodium acetate (CH3COO – Na +) in acetic acid under SN1. Identify the products.arrow_forwardDraw a reaction-energy diagram for the propagation steps of the free-radical addition of HBr to isobutylene. Draw curves representing the reactions leading to both the Markovnikov and the anti-Markovnikov products. Compare the values of ∆G° and Ea for the rate-limiting steps, and explain why only one of these products is observed.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
