
Concept explainers
(a)
Interpretation:
Balanced equation for the reaction between
Concept Introduction:
Chemical equation is the representation of a
According to physical and chemical properties, the elements can be further divided into metals, non-metals and metalloids.
In a group, the metallic character of an element increases from top to bottom whereas in a period, it decreases from left to right.
The classification elements in the periodic table as metals, nonmetals, or metalloids can be given as
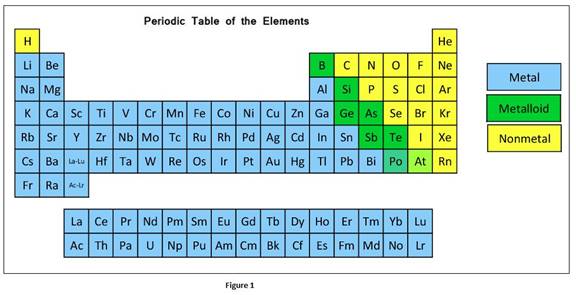
Oxide is the compound formed when oxygen reacts with another element. Oxides formed with metals are basic
Most of oxides formed with nonmetals are acidic.
Amphoteric oxides have the properties of bases and acid. Elements that are in the intermediate position of periodic table form amphoteric oxide.
(b)
Interpretation:
Balanced equation for the reaction between
Concept Introduction:
Chemical equation is the representation of a chemical reaction, in which the reactants and products of the reactions are represented left and right side of an arrow respectively by using their respective chemical formulas.
According to physical and chemical properties, the elements can be further divided into metals, non-metals and metalloids.
In a group, the metallic character of an element increases from top to bottom whereas in a period, it decreases from left to right.
The classification elements in the periodic table as metals, nonmetals, or metalloids can be given as
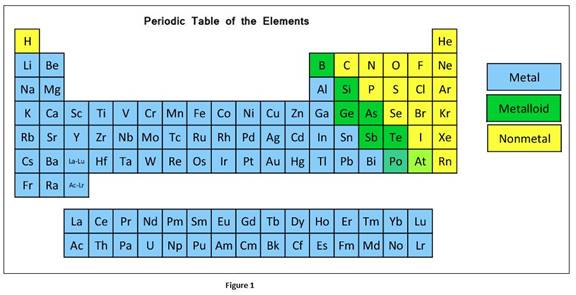
Oxide is the compound formed when oxygen reacts with another element. Oxides formed with metals are basic
Most of oxides formed with nonmetals are acidic.
Amphoteric oxides have the properties of bases and acid. Elements that are in the intermediate position of periodic table form amphoteric oxide.
(c)
Interpretation:
Balanced equation for the reaction between
Concept Introduction:
Chemical equation is the representation of a chemical reaction, in which the reactants and products of the reactions are represented left and right side of an arrow respectively by using their respective chemical formulas.
According to physical and chemical properties, the elements can be further divided into metals, non-metals and metalloids.
In a group, the metallic character of an element increases from top to bottom whereas in a period, it decreases from left to right.
The classification elements in the periodic table as metals, nonmetals, or metalloids can be given as
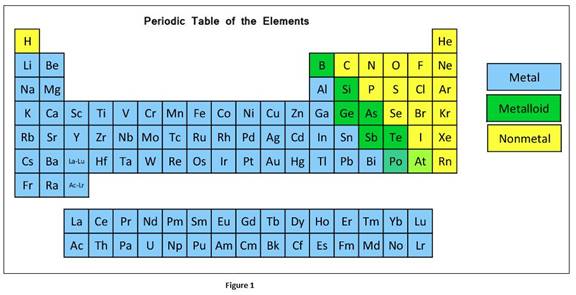
Oxide is the compound formed when oxygen reacts with another element. Oxides formed with metals are basic
Most of oxides formed with nonmetals are acidic.
Amphoteric oxides have the properties of bases and acid. Elements that are in the intermediate position of periodic table form amphoteric oxide.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
General Chemistry
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





