
The following data describe the binding of oxygen to human myoglobin at
From these data, estimate (a)
Hg, the partial pressure of
(a)
Interpretation: The value of
Concept Introduction: The hemoglobin capacity of oxygen is 1.34 mL per gram of oxygen gas. This capacity increases the total capacity of blood oxygen 70 times comparing to the dissolved oxygen in the blood. In mammals, the hemoglobin molecule can carry maximum of 4 oxygen molecules.
Explanation of Solution
The data given is as follows:
| 0.5 | 0.161 |
| 1 | 0.277 |
| 2 | 0.434 |
| 3 | 0.535 |
| 4 | 0.605 |
| 6 | 0.697 |
| 8 | 0.754 |
| 12 | 0.821 |
| 20 | 0.885 |
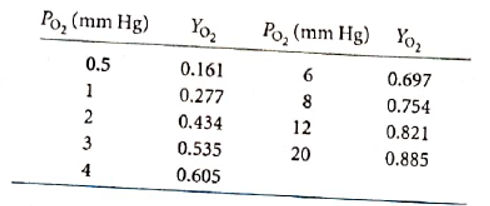
The graph between
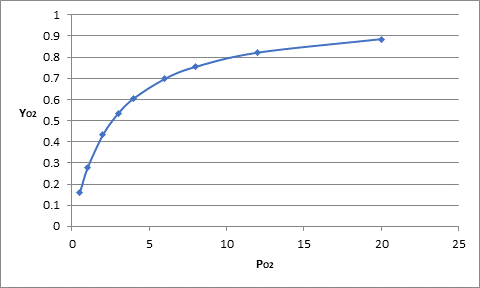
The value of
For the above graph, the trending line equation is represented as follows:
Putting the value of
Or,
Thus, the partial pressure is approximately 2.6 mm Hg.
(b)
Interpretation: The fraction of saturation of myoglobin at 30 mm Hg partial pressure of oxygen needs to be determined.
Concept Introduction: The hemoglobin capacity of oxygen is 1.34 mL per gram of oxygen gas. This capacity increases the total capacity of blood oxygen 70 times comparing to the dissolved oxygen in the blood. In mammals, the hemoglobin molecule can carry maximum of 4 oxygen molecules.
Explanation of Solution
The fraction of saturation at partial pressure 30 mm Hg is represented in the graph as follows:
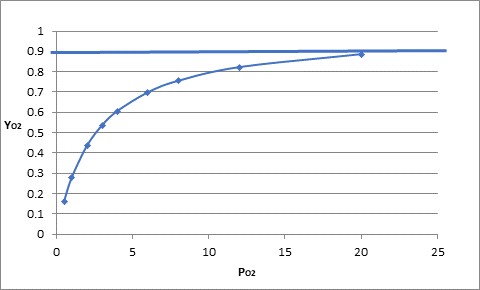
On extrapolated, the value of
Want to see more full solutions like this?
Chapter 7 Solutions
Biochemistry
- Below is the oxygen saturation curve for myoglobin and hemoglobin at a pH of 7. The p50 for myoglobin is indicated by the dashed lines on the graph. Mb and Hb O2 saturation: pH 7 10 0.8 Myoglobin 0.6 P50 = 0.2 0.4 Hemoglobin 0.2 - 0.0 pO2 [kPa] Which of these molecules (Mb/Hb/neither or both) has cooperativity? [ Select ] What would you expect to happen to the p50 of myoglobin if the pH were decreased to a pH of 4? [ Select ] Fraction saturationarrow_forwarda) How much more O2 can be transported by the blood when erythrocytesleave the lungs? Consider that a normal adult has a concentration of 15 g hemoglobin/100 mL of bloodand that the hemoglobin is 98% saturated with O2 at the usual pO2 of 100 torr in the lung at sea level. b) On the basis of the graph, explain how myoglobin facilitates the diffusion of O2 through muscle cells. Would myoglobin be effective as an O2-transport protein in cells of other tissues? Explain.arrow_forwardFrom the figure of O2 binding to myoglobin and hemoglobin (ignore the linemarked as T) as described in lecture (shown below) answer the following questions. a) Estimate the P50 for myoglobin from the plot. Show how this estimation isdetermined from the binding curve above. ( The first ghraph) b)Using YO2 = PO2/P50 + PO2 , calculate the fraction of O2 bound for myoglobin at 1 torr. (2nd graph) c)Using the binding curve on the previous page, show how you can estimate whatfraction of hemoglobin is bound near tissues at a pO2 of 30 torr and provide this value. If the pH were lowered, will the amount of O2 bound to hemoglobin at 30 torr increaseor decrease? Explain why this is so based on how this changes hemoglobin structure. If 2,3-BPG were added to the solution, will the amount of O2 bound to hemoglobin at30 torr increase or decrease? Explain why this is so based on how this changes hemoglobinstructure.arrow_forward
- Measurements of oxygen binding by whok human blood, at 37 °C, at pH 7.4, and in the presence of 40 mm Hg of CO, and normal physiological lkvels of 2,3-BPG (5 mmol/L of cells), give the following: Po, (mm Hg) % Saturation (=100 x Yo) 10.6 10 19.5 30 27.4 50 37.5 70 50.4 85 77.3 96 92.3 98 (a) From these data, construct a binding curve, and estimate the percent oxygen saturation of blood at (1) 100 mm Hg, the approximate partial pressure of O, in the lungs, and (2) 30 mm Hg, the approximate partial pressure of Oz in venous blood. (b) Under these conditions, what percentage of the oxygen bound in the lungs is delivered to the tissues? (c) Using the data in Figure 7.27, repeat the calculation of part (b) if the pH drops to 6.8 in capillaries but goes back to 7.4 as CO, is unloaded in the lungs.arrow_forward8.arrow_forwardStudies of oxygen transport in pregnant mammals show that the O2-saturation curves of fetal and maternal blood are markedly different when measured under the same conditions. Fetal erythrocytes contain astructural variant of hemoglobin, HbF, consisting of two α and two γ subunits (α2 γ2 ), whereas maternal erythrocytes contain HbA (α2β2).(a) Which hemoglobin has a higher affinity for oxygen under physiological conditions, HbA or HbF? Explain.(b) What is the physiological significance of the different O2 affinities?(c) When all the BPG is carefully removed from samples of HbA and HbF, the measured O2 -saturation curves (and consequently the O2 affinities) are displaced to the left. However, HbA now has a greater affinity for oxygen than does HbF. When BPG is reintroduced, the O2 -saturation curves return to normal, as shown in the graph. What is the effect of BPG on the O2 affinity of hemoglobin? How can the above information be used to explain the different O2 affinities of fetal and…arrow_forward
- A patient carrying a mutant form of hemoglobin (KD = 48 torr) is planning to take part in a hiking trip that involves strenuous physical activity at ~10,000 feet above sea level. The mutant hemoglobin has reduced oxygen binding cooperativity (n = 2.2) and displays no major structural abnormalities. a. Calculate the percent saturation of hemoglobin in the lungs (pO2 = 70 torr at this elevation) for this patient and for an individual carrying a normal version of hemoglobin b. Calculate the percent saturation of hemoglobin in active skeletal muscle tissue (pO2 = 15 torr) for this patient and for an individual carrying a normal version of hemoglobin c. Will this patient transport oxygen from the lungs to active muscle tissues more or less efficientlythan an individual with a normal version of hemoglobin on this trip? Briefly explain your answerarrow_forwardStudies of oxygen transport in pregnant mammals show that the O2-saturation curves of fetal and maternal blood are markedly different when measured under the same conditions. Fetal erythrocytes contain a structural variant of hemoglobin, HbF, consisting of two a and two y (a2 y2), whereas maternal erythrocytes contains HbA (a2 B2). 1.0 ·HbF +BPG 0.5 HbA +ВPG 2 4 6 8 10 pO2 (kPa) Which hemoglobin has a higher affinity for oxygen under physiological conditions, HbA or HbF? Explain. b. What is the physiological significance of the different O2 affinities?arrow_forwardA new oxygen transport protein that exhibits cooperative binding has been isolated and is beingstudied in the lab. Calculate the KD value if Y = 0.76 when pO2 = 18 torr (assume n = 2.5). Howdoes this compare to the KD value for hemoglobin? Does this protein bind more or less tightly tooxygen compared to hemoglobin?arrow_forward
- Studies of oxygen transport in pregnant mammals show that the O2-saturation curves of fetal and maternal blood are markedly different when measured under the same conditions. Fetal erythrocytes contain a structural variant of hemoglobin, HbF, consisting of two a and two y (a2 y2), whereas maternal erythrocytes contains HbA (a2 B2). 1.0 HbF +BPG e 0.5 HbA +ВPG 2 4 6 8 10 pO2 (kPa) What is the physiological significance of the different O2 affinities?arrow_forwardCalculate the percent oxygen saturation of myoglobin if 7 of 15 binding sites are occupied..arrow_forwardCompare the structure and function of hemoglobin and myoglobin. Which oxygen binding curve in the graph below belongs to myoglobin and which to hemoglobin? Please specify. Explain the effect of pH on oxygen binding to hemoglobin, and show on the graph in which direction the pH will shift if it falls below the physiological pH value (7.4).arrow_forward
 Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning

