
Review of Selected Concepts Related to Nomenclature
Write the chemical formula of each of the following. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
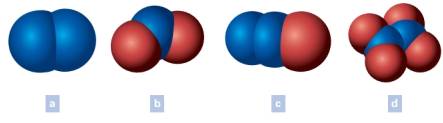
(a)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (a) is written as N2
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. The number of each kind of atom which makes up the particle generally called as the composition, is denoted by the chemical formula. Commonly, elements can be madeup of molecules having single atom, two atoms or complex multi-atoms. Coversely, when the substance itself is an element it should have all atoms of the same element. In the above example (a), the two lobes are having the same blue color which is for the element nitrogen.
Total number of lobes of nitrogen (N) = 2
Chemical formula = N2
Thus, the chemical formula of the colored lobes (a) is written.
(b)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (b) is written as NO2
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (b), the two lobes are having the same color which is for the element oxygen and one lobe is having the color for nitrogen.
Total number of lobes of nitrogen (N) = 1
Total number of lobes of oxygen (O) = 2
Chemical formula = NO2
Thus, the chemical formula of the colored lobes (b) is written.
(c)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (c) is written as N2O
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (c), the two lobes are having the same color which is for the element nitrogen and one lobe is having the color for oxygen.
Total number of lobes of nitrogen (N) = 2
Total number of lobes of oxygen (O) = 1
Chemical formula = N2O
Thus, the chemical formula of the colored lobes (c) is written.
(d)
Interpretation:
The chemical formula of each of the following is to be written. The blue spheres represent nitrogen atoms and the red spheres oxygen atoms. Oxygen is written last in the formulas that include oxygen.
Concept introduction:
The particle of an element or compound in the written format is denoted by the chemical formulas. The symbols of elements in a particular substance covers the formula of the required substance.
Answer to Problem 1E
The chemical formula of (d) is written as N2O4
Explanation of Solution
Generally, in a formula, the total number of atoms of the element under study is shown by a subscript number immediately following the symbol. Notably, the subscript does not have the number when only one atom of an element present in the formula. In the above example (d), the two lobes are having the same color which is for the element nitrogen and other four lobes are having the color for oxygen.
Total number of lobes of nitrogen (N) = 2
Total number of lobes of oxygen (O) = 4
Chemical formula = N2O4
Thus, the chemical formula of the colored lobes (d) is written.
Want to see more full solutions like this?
Chapter 6 Solutions
Introductory Chemistry: An Active Learning Approach
- What are the IUPAC names of the following compounds? manganese dioxide mercurous chloride ( Hg2Cl2) ferric nitrate [Fe( No 3)3] titanium tetrachioride cupric bromide (CuBr2)arrow_forwardTell what is wrong with each of the following molecular formulas and write a correct formula: a. H3PO3 phosphorous acid b. SICI4 silicon tetrachloride c. SOO sulfur dioxide d. 2HO hydrogen peroxide-two hydrogen atoms and two oxygen atomsarrow_forwardWhat is wrong with the formula "HgCl" for mercury (I) chloride? Group of answer choices There is nothing wrong with this formula Mercury (I) must always be paired with another mercury (I) Mercury does not commonly exist in the +1 form The formula should be Hg2Cl Mercury never forms compounds with other elements; this shouldn't existarrow_forward
- Which of the following is NOT a correct name? (five are correct names for any compound, but one is NOT correct for any compound) Group of answer choices lithium dihydrogen phosphate copper (I) bromide potassium bicarbonate calcium hydrogen carbonate magnesium hypochlorite dicalcium phosphatearrow_forwardGive the name of each of the following polyatomic anions. a.CO32d.PO43 b.ClO32e.ClO4 c.SO42f.MnO4arrow_forwardName each of the following binary compounds, using the periodic table to determine whether the compound is likely to be ionic (containing a metal and a nonmetal) or nonionic (containing only nonmetals), MgSd.ClBr AlCl3e.Li2O PH3f.P4O10arrow_forward
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning





