
Determine the point groups for
a. Ethane (staggered conformation)
b. Ethane (eclipsed conformation)
c. Chloroethane (staggered conformation)
d. 1,2-Dichloroethane (staggered anti conformation)
(a)
Interpretation:
The point group for Ethane with staggered conformation is to be determined.
Concept Introduction :
A point group defines all the operations of symmetry that could be performed on a molecule, results in an indistinguishable conformation from the original.
Answer to Problem 4.1P
The point group of ethane with staggered conformation is D3d.
Explanation of Solution
The structure of ethane in staggered conformation can be drawn as
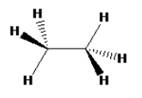
Ethane with staggered conformation has a C3 rotational axis that passes through C-C bond. There are 3 C2 rotational axes that will be perpendicular to the C3 rotational axis. It passes through the mid point of the C-C bond and doesnot have any
(b)
Interpretation: The point group for Ethane with eclipsed conformation is to be determined.
Concept Introduction:
A point group defines all the operations of symmetry that could be performed on a molecule, results in an indistinguishable conformation from the original.
Answer to Problem 4.1P
The point group of ethane with eclipsed conformation is D3h.
Explanation of Solution
The structure for ethane in eclipsed conformation is
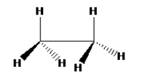
C3-rotational axis that passes through C-C bond is present in ethane in eclipsed conformation. This conformation has three C2 axes which are perpendicular to the principal C3 rotational axes and it passes through the mid-point of the C-C bond. The point group of the eclipsed ethane molecule is D3h as it has a plane of
(c)
Interpretation: The point group for chloroethane with staggered conformation is to be determined.
Concept Introduction : A point group defines all the operations of symmetry that could be performed on a molecule, results in an indistinguishable conformation from the original.
Answer to Problem 4.1P
The point group in chloroethane with staggered conformation is Cs.
Explanation of Solution
The structure of Chloroethane with staggered conformation can be drawn as
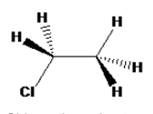
The staggered conformation of chloroethane do not have any rotational axis.
(d)
Interpretation:
The point group for 1,2-dichloroethane with staggered anti-conformation is to be determined.
Concept Introduction:
A point group defines all the operations of symmetry that could be performed on a molecule, results in an indistinguishable conformation from the original.
Answer to Problem 4.1P
The point group of 1,2-dichloroethane with staggered anti-conformation is C2h.
Explanation of Solution
The staggered anti-conformation of 1,2-dichloroethane has C2 rotational axis and it passes through the mid-point of C-C bond. This is perpendicular to the plane of Cl-C-C-Cl. The point group of the molecule is C2h as it has only one
Want to see more full solutions like this?
Chapter 4 Solutions
Inorganic Chemistry
Additional Science Textbook Solutions
Human Biology: Concepts and Current Issues (8th Edition)
Chemistry (7th Edition)
Human Physiology: An Integrated Approach (8th Edition)
Applications and Investigations in Earth Science (9th Edition)
Chemistry: An Introduction to General, Organic, and Biological Chemistry (13th Edition)
Genetic Analysis: An Integrated Approach (3rd Edition)
- Predict the major organic product of the reaction. H₂N. 1 eq. Ac₂O OH Select Draw Templates More C H N Erase +ACO¯arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardGiven the following reactions show the mechanism by which electrons are moved using curly arrow notation. H Br H20 Na OCH 3 CH₂OM, 50°C H OHarrow_forward
- Question 3: What is the bond order in H₂¹? 02 1 O 1.5 0 ○ 0.5 Check Reuse Embed H-Parrow_forwardDraw the product of the reaction shown below. Ignore inorganic byproducts. HзN. |||| + CBz-Gly DCCarrow_forwardConsider a system of two energy levels separated by an energy equal to ε1. The lowest energy level is nondegenerate, and the second energy level has a degeneracy of 4. a) Write the expression for the partition function. b) Write the expression for E. Determine the value of E in the infinite temperature limit. c) Write the expression for S. Determine the value of S in the infinite temperature limit.arrow_forward
- Question 4: -1 Molecular orbital theory would predict H21 is cannot predict paramagnetic diamagnetic Check Reuse Embed H-Parrow_forwardCyclooctylmethanol + ch3ch2mgbrarrow_forward3 Energy (EU) Set A Set B Set C 2 1 2 4 4 1 8 11 329 3 2 9 0 16 14 16 a) Demonstrate that the sets have the same energy. b) Determine which of the sets is the most probable. c) For the most probable set, is the energy distribution consistent with a Boltzmann distribution? Explain your answer.arrow_forward
- Consider a system of two nondegenerate energy levels separated by 3000 cm-¹. Measurement of the level populations demonstrates that there are 6 times more molecules in the ground state than in the upper state. What is the temperature of the collection?arrow_forwardPlease correct answer and don't used hand raitingarrow_forwardEnergy A sample consisting of 4 molecules has a total energy of 6 EU. There are four possible energy states corresponding to 0 EU, 1 EU, 2 EU and 3 EU, which is illustrated by the following figure. E = 3 EU E = 2 EU E = 1 EU E = 0 EU a) Determine all possible configurations, and determine the weight and probability of each configuration. b) Which configuration(s) is (are) most probable? c) Which configuration(s) is least probable?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

