
Concept explainers
Interpretation:
The structure of compound X formed by the reaction of
Concept Introduction:
Sodium borohydride is a reducing agent which reduces carbonyl group to the alcohol group. Ribitol and Xylitol are both five carbon alditols. Periodic acid is a strong oxidizing agent which converts alcohol
Answer:
The structure of X is shown below.
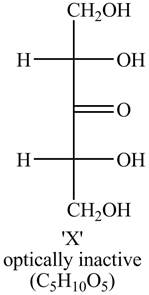
Explanation:
As X gives ribitol and xylitol on reduction with sodium borohydride, so, the oxidation of both alditols performed at carbon
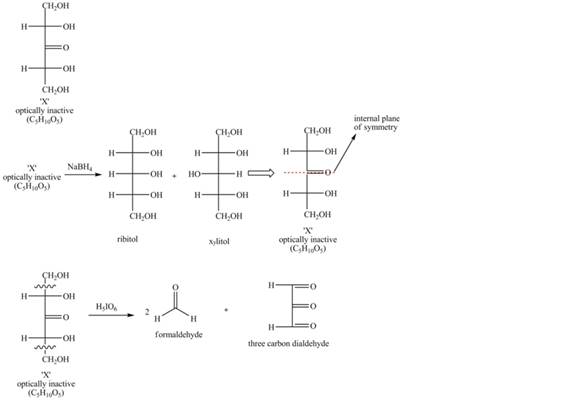
Figure 1
Conclusion:
The structure of compound X is shown in Figure 1.
Want to see the full answer?
Check out a sample textbook solution
Chapter 24 Solutions
EBK ORGANIC CHEMISTRY
- What is the anhydride for each of the following acids:(a) H2SO4, (b) HClO3, (c) HNO2, (d) H2CO3, (e) H3PO4?arrow_forwardIt is well known that humans are able to digest amylose, but not cellulose. What structural features might be responsible this observation?arrow_forwardPlease explain in detail and give the name for all the compunds tooarrow_forward
- 27. Which of the following statements about cholesterol is not correct? CH3 CH3 H. d но Cholesterol (a) Cholesterol is a steroid that contains a tetracyclic ring system. (b) Cholesterol is a steroid that contains 8 chiral carbons and can form 28 or 256 stereoisomers. (c) Each atom or group attached to a ring-junction carbon (i.e., carbons a -e) is in a trans or axial position. Because of this the tetracyclic ring system is mostly flat. (d) Cholesterol is used to synthesized vitamin D, bile acids, sex hormones, and adrenocorticoid hormones. (e) Cholesterol is not found in the cell membranes of animals.arrow_forwardPlease explain and give the name of the compounds tooarrow_forward(a) What amino acids would be obtained by hydrolysis of the following tripeptide? H,NCHCNHCHCNHCHCOH (CH)2CH H.CоН Н-ССH-СОН (b) How many different tripeptides can be made from gly- cine, serine, and glutamic acid? Give the abbreviation for each of these tripeptides, using the three-letter codes and one-letter codes for the amino acids.arrow_forward
- The structural formula for the open-chain form of galactose is CH Н—ҫ—ОН Но—С—н НО—С—Н Н—ҫ—ОН CH-ОН (a) Is this molecule a sugar? (b) How many chiral carbons are present in the molecule? (C) Draw the structure of the six-member-ring form of this molecule.arrow_forwardGive the chemical equation of the following reactions:arrow_forwardGlutamic acid has three ionizable groups. The pKa of α-COOH is 2.19; a-NH 3+, 9.67; and the R-group, 4.25. What is the charge of glutamic acid at pH 10.00?arrow_forward
- Draw the structure of (a) a ketotetrose; (b) an aldopentose; (c) an aldotetrose.arrow_forwardB) Which of the compounds pictured could be identified as one controlling the development of the sex organs and secondary sexual characteristics? Would it react with NaBH4? Explain why or why not.arrow_forwardShow how to convert the side-chain carboxyl group to a benzyl ester using benzyl chloride as a source of the benzyl group.arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





