
EBK ORGANIC CHEMISTRY
12th Edition
ISBN: 9781119233664
Author: Snyder
Publisher: VST
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 23, Problem 2PP
Interpretation Introduction
Interpretation:
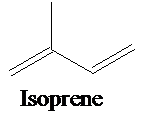
The isoprene units in the given compounds are to be shown and each of them as per their isoprene units are to be classified.
Concept introduction:
Essential oils are mixtures of odoriferous compounds that are extracted from plants by heating or steam distillation. These essential oils have important chemical composition of hydrocarbons called terpenes and oxygen-containing compounds called terpenoids.
A single isoprene unit consists of 5-carbon atoms with double bonds, and its chemical formula is
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Ciprofloxacin is a member of the fluoroquinolone class of antibiotics.(a) Which of its rings are aromatic?
Answer ALL parts of this question.
(a) Provide the structure and name of the aldehyde with the formula, C2H4O.
(b) Draw the two steps of the Strecker synthesis used for the conversion of the
aldehyde in part (a) into racemic alanine.
(c) Draw the (R) and (S) enantiomers of alanine. Which is the naturally occurring
enantiomer?
(d) Draw the structure of the dipeptide, Ala-Gly.
(a) Give chemical tests to distinguish between compounds in the following pairs of substances :(i) Ethanol and Propanal (ii) Benzoic acid and Ethyl benzoate(b) An organic compound contains 69.77% carbon, 11.63% hydrogen and rest oxygen. The molecular mass of the compound is 86. It does not reduce Tollen’s reagent but forms an addition compound with sodium hydrogen sulphite and gives positive iodoform test. On vigorous oxidation it gives ethanoic and propanoic acids. Derive the structure of the compound.
Chapter 23 Solutions
EBK ORGANIC CHEMISTRY
Ch. 23 - Prob. 1PPCh. 23 - Prob. 2PPCh. 23 - Prob. 3PPCh. 23 - Prob. 4PPCh. 23 - Prob. 5PPCh. 23 - Prob. 6PPCh. 23 - Prob. 7PPCh. 23 - Prob. 8PPCh. 23 - Prob. 9PPCh. 23 - Prob. 10PP
Ch. 23 - Prob. 11PPCh. 23 - Prob. 12PPCh. 23 - Prob. 13PPCh. 23 - Prob. 14PCh. 23 - 23.15 How would you transform tetradecanal into...Ch. 23 - Prob. 16PCh. 23 - Prob. 17PCh. 23 - When limonene (Section 23.3) is heated strongly,...Ch. 23 - Gadoleic acid (C20H38O2), a fatty acid that can be...Ch. 23 - 23.20 -Phellandrene and -phellandrene are isomeric...Ch. 23 - Prob. 21PCh. 23 - Prob. 22PCh. 23 - Prob. 23PCh. 23 - The initial steps of a laboratory synthesis of...Ch. 23 - Prob. 25PCh. 23 - Prob. 26PCh. 23 - Prob. 27PCh. 23 - 2. The biosynthesis of fatty acids is accomplished...Ch. 23 - Prob. 3LGPCh. 23 - Prob. 4LGP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (a) Differentiate between copolymerization and homopolymerization. Give one example of each.(b) What is the role of Benzoyl peroxide in preparation of Polythene?arrow_forwardAspirin, or 2-acetoxybenzoic acid, (C9H8O4) is often synthesised from salicylic acid.(i) Sketch and discuss any changes in the number of possible structural conformations ofaspirin relative to those of salicylic acid. (ii) Re-draw the structure predicted to be the lowest energy conformation of aspirin,indicating any expected stabilising and destabilising interactions. Justify your choice.arrow_forwardb) Disaccharide E is a reducing sugar. It is hydrolyzed by an α-glycosidase enzyme, which means it contains an α- glycoside link. Treatment of E with Ag2O and excess Mel gives an octamethyl derivative F. Hydrolysis of F in dilute aqueous acid gives the pair of molecules shown below. Write the structures of E and F. (If the stereochemistry at a particular carbon is not determined by the above data, indicate this with a wavy line as shown below.) HO OMe OMe Is is MeO MeO MOH OMe mOH OMe OMearrow_forward
- Show the chemical reaction on how to convert cyclopentene into these compounds. (a) 1,2-dimethylcyclopropane (b) Cyclopentanol (c) Iodocyclopentane (d) Cyclopentane.arrow_forwardKindly answer this questionarrow_forwardGive reasons :(i) Electrophilic substitution in aromatic amines takes place more readily than benzene.(ii) CH3CONH2 is weaker base than CH3CH2NH2.arrow_forward
- Give reasons: (i) Bond length of C = O in carboxylic acids is slightly larger than C = O bond length in carbonyl compounds. (ii) There are two –NH2 groups in semicarbazide. However, only one –NH2 group is involved in the formation of semicarbazones. (iii) Benzoic acid is less soluble in water than acetic acid. (iv) Formic acid is a stronger acid than acetic acid.arrow_forward(a) Write the structures of the following compounds and mark them as chiral or achiral. 4 (i) 2-Bromopentane (ii) 3-Bromopentane (iii) 1-Bromo-2-methylbutane (iv) 2-Chloro-3-methylbutane (b) Identify the asymmetric carbon in the chiral compounds. (c) Write the structure of the other enantiomer of the chiral compounds.arrow_forwardb) Disaccharide E is a reducing sugar. It is hydrolyzed by an α-glycosidase enzyme, which means it contains an α- glycoside link. Treatment of E with Ag2O and excess MeDgives an octamethyl derivative F. Hydrolysis of F in dilute aqueous acid gives the pair of molecules shown below. Write the structures of E and F. (If the stereochemistry at a particular carbon is not determined by the above data, indicate this with a wavy line as shown below.) HO OMe OMe MeO MeO OH OMe Am OH OMe OMearrow_forward
- Compund ( A) C6H12 is treated with chlorine in the presence of carbon tetrachloride to given (B)C6H12Cl2.Compund (B) is treated with alcoholic KoH,followed by NaNH2 ,resulting in this formation of (C).compound (C) is treated with hydrogen in the presence of nickel catalysts to give 2methylpentane. Compound (C) does not react with sodamide or ammonical solution of silver nitrate .Ozonolysis of (A) give two aldehyde, (D) and (E) .Compound (E) is identified as acetaldehyde .From this information,describe the structural formula (A) ,(B) ,(C) And (C).write equation for all reactionarrow_forwardb) i) Draw the different structural isomers of the 6-membered ring compound [GAAIBNPAS]H6 in which there are alternating Group 13 and 15 elements. ii) The isomers containing both B-N and AI-N bonding are the most stable. Explain why this is the case. ii) How would you expect the structure and reactivity of the heterocycles [GAAIBNPAS]H6 to compare with borazine? iv) While borazine exists as a planar ring compound, the [GAAIBNPAS]H6 ring compounds dimerize. Explain these observations. H -ЕН -E H-E НЕ ЕН E НЕ- ЕН H `E H. H É-H НЕ E = Heteroatom エー山、 w-Iarrow_forward(b) Draw the structural formula for each of the following compounds. Lukis formula struktur bagi setiap sebatian berikut. (i) 3-isopropyl-3,4-dimethylhexanol (ii) 2-methyl-3-phenylprop-2-enal (iii) N-ethyl-N-methylbutanaminearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
