
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
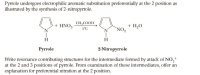
Transcribed Image Text:Pyrrole undergoes electrophilic aromatic substitution preferentially at the 2 position as
illustrated by the synthesis of 2-nitropyrrole.
CH,COOH
+ HNO3
+ H,O
`NO2
5°C
H
Рyrrole
2-Nitropyrrole
Write resonance contributing structures for the intermediate formed by attack of NO,+
at the 2 and 3 positions of pyrrole. From examination of these intermediates, offer an
explanation for preferential nitration at the 2 position.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Similar questions
- Please don't provide handwriting solution Which of the following compounds can undergo nucleophilic aromatic substitution reactions?arrow_forwardMake a systematic process for the synthesis of the benzene derivative from benzene. Present all the needed reagent and reaction conditions and products. (Note the electron-donating or electron- withdrawing) NH2arrow_forwardIsoerythrogenic acid, C18H26O2, is an acetylic fatty acid that turns a vivid blue on exposure to UV light. On Catalytic hydrogenation over a palladium catalyst, five molar equivalents of hydrogen are absorbed, and stearic acid, CH3(CH2)16CO2H, is produced. Ozonolysis of isoerythrogenic acid yields the following products: formaldehyde, CH2O, malonic acid, HO2CCH2CO2H, adipic acid, HO2C(CH2)4CO2H, and the aldehyde carboxylic acid, OHC(CH2)6CO2H. Provide a structure for isoerythrogenic acid.arrow_forward
- A common illicit synthesis of methamphetamine involves an interesting variation of the Birch reduction. A solution of ephedrine in alcohol is added to liquid ammonia, followed by several pieces of lithium metal. The Birch reduction usually reduces the aromatic ring, but in this case it eliminates the hydroxy group of ephedrine to give methamphetamine. Propose a mechanism, similar to that for the Birch reduction, to explain this unusual course of the reaction.arrow_forwardIn the reaction between 3-pentanone and 1-propanol in the presence of acid catalyst, the resulting FINAL acetal product has bonded to the four different sides of the central carbon. (NOTE: The numbers in the responses should be subscripted.) -СН2CH3, -СН2CНЗ, -ОСН2СНЗ, and -OCH2CНЗ -(CH2)4CH3, -(CH2)4CH3, -OCH2CH2CH3, and -OCH2CH2CH3 О -н, -СН2СHЗ, -ОСН2СH2CНЗ, and -OCH2CH2CH3 -CН2CH3, -СH2СНЗ, -ОСН2СH2CH3, and -OСH2СH2CH3 -CH2CH3, -CH2CH3, -OCH2CH2CH3, and -OHarrow_forwardChemistry Please help explain this textbook question: Although N, N -dimethylaniline is extremely reactive toward electrophilic aromatic substitution and is readily substituted by weak electrophiles, such as diazonium and nitronium ions, this reactivity is greatly diminished by the introduction of an alkyl substituent in an ortho position.arrow_forward
- Give the structure of the organic product formed after the following series of reactions. Br Gee die struktuur van die organiese produk wat gevorm word na die volgende reeks van reaksies. 1) Mg 2) 4-methylpentanal | 4-metielpentanaal 3) PBr3 4) propylamine | propielamien 5) NaHCO3arrow_forwardEnamines can serve as enolate surrogates in reactions at the a-carbon. In the reaction sequence, the structures of the enamine addition product – the initial zwitterion and its neutral tautomer – are shown. Draw the structures of the two reactants forming these intermediates, and draw the structure of the final product, obtained via hydrolysis of the neutral intermediate. initial zwitterionic intermediate neutral intermediate tautomerization Reactants H,0 hydrolysis product Draw the two reactants. Draw the hydrolysis product. Select Draw Rings More Erase Select Draw Rings More Erase H Harrow_forwardSuggest a strategy for converting 1-methylcyclohexene into 1-cyclohexylmethanol. Provide the structures for the major organic products frormed in each step.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY