
Concept explainers
Fill the blanks in the following table.
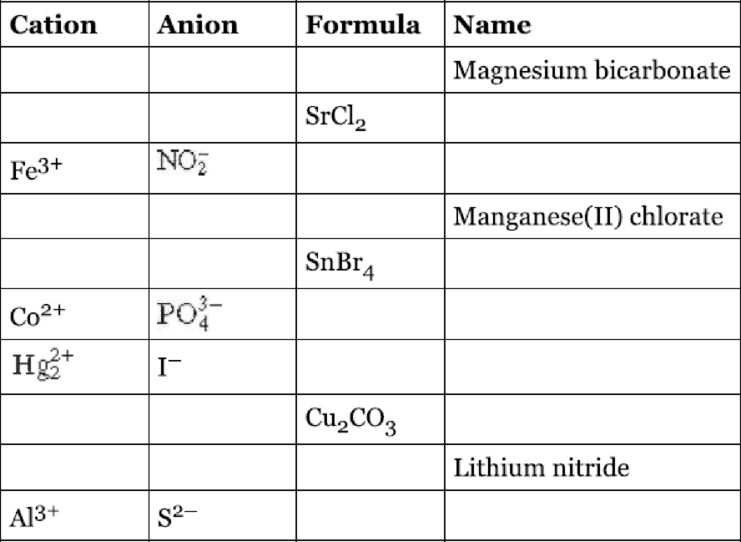
Interpretation:
The given table has to be filled.
Explanation of Solution
The given name is Magnesium bicarbonate. The cation and anion present in the compound are
The given formula is
The cation and anion present in the compound is
The given name is Manganese
The given formula is
The cation and anion present in the compound are
The cation and anion present in the compound are
The given formula is
The given name is Lithium nitride. The cation and anion present in the compound are
The cation and anion present in the compound are
The completed table is shown below,
| Cation | Anion | Formula | Name |
| Magnesium bicarbonate | |||
| Strontium chloride | |||
| Iron | |||
| Manganese | |||
| Tin | |||
| Cobalt | |||
| Mercury | |||
| Copper | |||
| Lithium nitride | |||
| Aluminum sulphide |
Want to see more full solutions like this?
Chapter 2 Solutions
Chemistry
- 7. (10 points) Indicate the type of crystal (molecular, metallic, ionic, or covalent-network) each of the following would form upon solidification a. Na2CO3 molecular metallic ionic covalent-network b. Cu molecular metallic ionic covalent-network C. acetone molecular metallic ionic covalent-network d. Mg molecular metallic ionic covalent-network 5004 e. C molecular metallic ionic covalent-network 49arrow_forwardThe balanced chemical reaction of aluminum with oxygen is shown below. reaction A 26.98g sample of Al is reacted with excess oxygen (O2) yielded 24.82-g Al2O3. What is the percent yield of this reaction? 4Al(s) + 3O2(g) → 2Al2O3(s) Al 26.98g/mol O2 32.00g/mol Al2O3 101.96g/molarrow_forward2) a) Draw the product if Molecule A underwent a [3,3]-sigmatropic rearrangement. Hint, you may want to start by redrawing the starting materials in their reactive conformations. (4 points) Molecule A CH3 CH2 b) Show the complete, step-wise curved arrow mechanism for the above reaction. You may use H-B for any Broensted acid and B- for any Broensted base. You can maximize your chances of partial credit by providing a consistent numbering scheme for your carbons. (9 points)arrow_forward
- 1) A reaction is proposed to take place in the following elementary steps. (1) AB + C → D(2) D + C → E(3) E + AB → F If the rate law for this reaction was found experimentally to be: Rate = k [AB]2[C]2, which step must be the slowstep?arrow_forwardPlease help me with 8,9,10 & 14 based on the data I've provided. Thanks so much in advance!arrow_forward1.60 atm PCl5 is placed in a flask to decompose. What are the concentrations at equilibrium, given the total pressure is 1.80 atm? PCl5(g)↔ PCl3(g) + Cl2(g)arrow_forward
- jpsarrow_forward6.2-Methylnaphthalene can be synthesized from toluene through the following sequence of reactions. Write the structure of each intermediate. Toluene + AICI, A (C₁, H₁₂O₂) Zn(Hg) HCI B (C₁,H₁₂O₂) SOCI AICI₂ NaBH C (C₁₁H₁₂CIO) »D (C₁H₁₂O) E (C,,H₁₂O) H₂SO4 NBS NaOEt F (C,,H₁₂) heat CCI, light G (C,,H₁₂Br) EtOH, heatarrow_forward6.16 Before solving the problem please also give a brief explanation of the concept or associated equation(s) and variables. (DO NOT FORGET K-1 AND K2 I believe steady state approximation will be important in solving this problem) (Answer I believe should be r = k2[A][B] r = {k2 (k1/k-1)1/2}[A2]1/2[B])arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning





