
Concept explainers
- The numbers of orbiting electrons in aluminum and silver are 13 and 47, respectively. Draw the electronic configuration for each, and discuss briefly why each is a good conductor.
- Using the Internet, find the atomic structure of gold and explain why it is an excellent conductor of electricity.
(a)
To draw:
The electronic configuration of aluminum and silver. Also, state the reason due to which both aluminum and copper are considered a good conductor.
Explanation of Solution
Given:
The numbers of orbiting electrons in aluminum and silver are
The arrangement of electron inside the atom and outside the nucleus follows a particular pattern. There is a shell structure in which all electron falls and the energy of the electrons decide which shell to fall. The number of electrons in each shell is always
In shell
In Shell
Inside shell, actually electron falls in sub-shell. The name of sub-shell are
In a nutshell, the larger the value of
The electronic configuration of any element is based on below pattern:
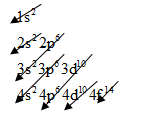
For aluminum, we know that the numbers of orbital electrons are 13.
So, arrange the electrons will be as below
The electronic configuration of Aluminum is
Here we can see, Aluminum has one free electron in its valence shell. Therefore, this free electron can move easily and hence, this causes the higher conductivity for Aluminum.
For silver, we know that the numbers of orbital electrons are 47.
So, arrange the electrons will be as follows:
The electronic configuration of silver is
Silver is a good conductor because it has free electrons in its valence shell. It is clearly seen from the electronic configuration of silver (
(b)
The atomic structure of the gold. Also, state the reason due is an excellent conductor of electricity.
Explanation of Solution
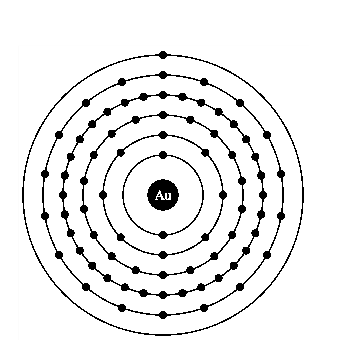
Atomic structure of gold.
The electronic configuration of any element is based on below pattern:
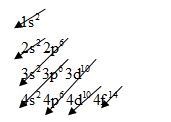
For gold, we know that the numbers of orbital electrons are
So, arrange the electrons for gold will be as follows:
The electronic configuration of gold is
Gold is a good conductor of electricity because it has free electrons in its valence shell.
It is clearly seen from the electronic configuration of gold that, s orbit is holding only one electron and maximum limit is up to
Want to see more full solutions like this?
Chapter 2 Solutions
Introductory Circuit Analysis (13th Edition)
Additional Engineering Textbook Solutions
Starting Out With Visual Basic (8th Edition)
Thinking Like an Engineer: An Active Learning Approach (4th Edition)
Java: An Introduction to Problem Solving and Programming (8th Edition)
Degarmo's Materials And Processes In Manufacturing
Web Development and Design Foundations with HTML5 (8th Edition)
Concepts Of Programming Languages
- 2) The transistor parameters of the NMOS device in the common-gate amplifier in Figure 2 are VTN = 0.4V, K'n = 100 μA / V², and λ=0. (50 points) a) Find RD such that VDSQ = VDs (sat) + 0.25V. b) Determine the transistor W/L ratio such that the small-signal voltage gain is Av=6. c) What is the value of VGSQ? Сс 2 mA Rp T V=-1.8 V V+= 1.8 V Figure 2arrow_forwardCalculate the percent voltage regulation for a three-phase wye-connected 2500 kVA 6600-V turboalternator operating at full-load Unity power factor The per phase synchronous reactance and the armature resistance are 10.4 2 and 0.071 ≤2, respectively?arrow_forwardDon't use ai to answer I will report you answerarrow_forward
- Chose the correct answer: 1- A squirrel cage induction motor is not selected when (A) initial cost is the main consideration (B) maintenance cost is to be kept low (C) higher starting torque is the main consideration (D) all above considerations are involved 2- The torque of an induction motor is .............. (A) directly proportional to slip (B) inversely proportional to slip... (C) proportional to the square of the slip (D) none of the above 3- Insertion of resistance in the stator of an induction motor. (A) increases the load torque (B) decreases the starting torque (C) increases the starting torque (D) none of above tool to slip 10 or of the above 4- Increase in the length of air-gap in the induction motor results in the increasing of its (A) air-gap flux (B) magnetizing current (C) speed (D) power factor 5- In cumulatively cascade method for speed controlling, if PA is the number of poles of main motor and PB is the number of poles of auxiliary motor. Then the speed of the set…arrow_forwardChose the correct answer: 1- The resultant flux in stator winding of three-phase induction motor is equal to (A) Maximum value of flux due to any phase (B) Twice of the maximum value of flux due to any phase. (C) 0.5 times the maximum value of flux due to any phase (D) 1.5 times the maximum value of flux due to any phase 2- Which one of the following starters cannot be used for 3-phase, star - connected, slip-ring induction motor? (A) Auto-transformer starter (B) Star-delta starter (C) Direct-on-line starter (D) Rotor resistance starter 3- The crawling in the induction motor is caused by.............. (A) low voltage supply (B) high loads (D) improper design of the machine (C) harmonics developed in the motor 4- The 'cogging' of an induction motor can be avoided by........... (A) good ventilation (B) using DOL starter (C) star-connecting of stator winding (D) having number of rotor slots more or less than the number of stator slots 5- The method which can be used for the speed control…arrow_forwardManual solution only, no Al usedarrow_forward
 Electricity for Refrigeration, Heating, and Air C...Mechanical EngineeringISBN:9781337399128Author:Russell E. SmithPublisher:Cengage Learning
Electricity for Refrigeration, Heating, and Air C...Mechanical EngineeringISBN:9781337399128Author:Russell E. SmithPublisher:Cengage Learning Delmar's Standard Textbook Of ElectricityElectrical EngineeringISBN:9781337900348Author:Stephen L. HermanPublisher:Cengage Learning
Delmar's Standard Textbook Of ElectricityElectrical EngineeringISBN:9781337900348Author:Stephen L. HermanPublisher:Cengage Learning

