
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15.2, Problem 5P
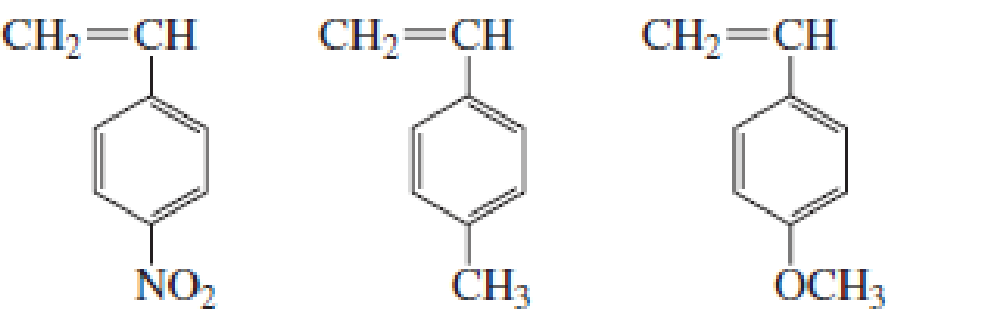
List the following groups of monomers in order from most able to least able to undergo cationic

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) Which monomer is most likely to undergo anionic polymerization? Justify your choice.
( b)Which one ismost likely to undergo cationic polymerization? Justify your choice.
Classify each polymer below as step-growth or chain-growth. Place the appropriate term in the
blank provided.
CH₂-CH
NH2
Dry-erase boards, or whiteboards, in classrooms consist
of a network polymer similar to Bakelite that is made
from formaldehyde and melamine. (a) Propose a
structure for the polymer formed. (b) Why is this type of
polymer suitable for use in whiteboards?
'N.
H2N
`NH2
Melamine
Chapter 15 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 15.2 - Prob. 1PCh. 15.2 - Prob. 2PCh. 15.2 - Prob. 3PCh. 15.2 - Prob. 4PCh. 15.2 - List the following groups of monomers in order...Ch. 15.2 - List the following groups of monomers in order...Ch. 15.2 - Prob. 7PCh. 15.2 - Why does methyl methacrylate not undergo cationic...Ch. 15.2 - Which monomer and which type of initiator would...Ch. 15.2 - Prob. 10P
Ch. 15.2 - Prob. 11PCh. 15.5 - Draw a short segment of gutta-percha.Ch. 15.5 - Prob. 13PCh. 15.6 - Prob. 14PCh. 15.8 - Prob. 15PCh. 15.8 - Prob. 16PCh. 15.8 - Prob. 17PCh. 15.8 - a. Propose a mechanism for the formation of the...Ch. 15.8 - Propose a mechanism for the formation of Melmac.Ch. 15.8 - Explain why, when a small amount of glycerol is...Ch. 15.10 - Prob. 21PCh. 15 - Draw short segments of the polymers obtained from...Ch. 15 - Prob. 23PCh. 15 - Draw the structure of the monomer or monomers used...Ch. 15 - Draw short segments of the polymers obtained from...Ch. 15 - Prob. 26PCh. 15 - Prob. 27PCh. 15 - Prob. 28PCh. 15 - A particularly strong and rigid polyester used for...Ch. 15 - Prob. 30PCh. 15 - Prob. 31PCh. 15 - Prob. 32PCh. 15 - Prob. 33PCh. 15 - Prob. 34PCh. 15 - Prob. 35PCh. 15 - Delrin (polyoxymethylene) is a tough...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider monomers A–C. (a) Rank the monomers in order of increasingreactivity in cationic polymerization. (b) Rank the monomers in order ofincreasing reactivity in anionic polymerization.arrow_forwardA3 Anionic polymerization of styrene is generally known to produce polymers with a narrow molecular weight distribution. Explain the reason for this.arrow_forwardRank the following compounds in order of increasing ability to undergo anionic chain-growth polymerization. ÇN LOCH3 OCH3arrow_forward
- Explain the effects the addition of glycerol (a triol) will have on the molecular architecture of the following polymerization reactions: a) Condensation polymerization of terephthalic acid + ethylene glycol b) Condensation polymerization of 6-hydroxyhexanoic acidarrow_forward(d) Another variation on this type of polymer is used in hair gels. In these, the polymer chains are cross-linked by a compound known as pentaerythritol. но- OH но- OH pentaerythritol ( By what type of chemicat reaction are the cross-links in this polymer formed? (i) it is important that the gel should be easily washed out of hair What is it about the structure of the polymer that allows this to happen?arrow_forwardWhat two monomers are needed to prepare each polymer?arrow_forward
- One common type of cation exchange resin is prepared by polymerization of a mixture containing styrene and 1,4-divinylbenzene . The polymer is then treated with concentrated sulfuric acid to sulfonate a majority of the aromatic rings in the polymer. Q.) Explain how this sulfonated polymer can act as a cation exchange resinarrow_forward(please answer within 20-30min)arrow_forwardOne common type of cation exchange resin is prepared by polymerization of a mixture containing styrene and 1,4-divinylbenzene . The polymer is then treated with concentrated sulfuric acid to sulfonate a majority of the aromatic rings in the polymer. Q.) Show the product of sulfonation of each benzene ring.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
CBSE Class 12 Chemistry || Polymers || Full Chapter || By Shiksha House; Author: Best for NEET;https://www.youtube.com/watch?v=OxdJlS0xZ0Y;License: Standard YouTube License, CC-BY