
(a)
Interpretation:
The systematic name for the below given compound has to be given.
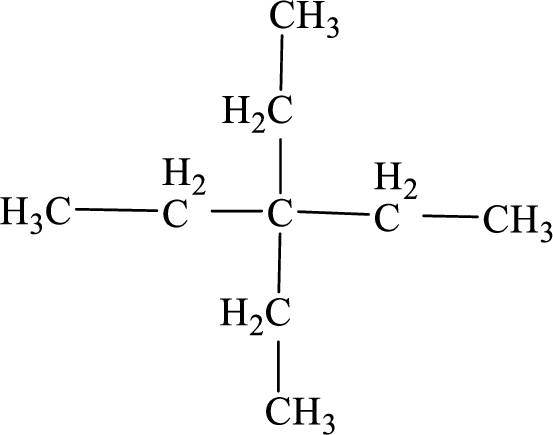
Concept Introduction:
The hydrocarbons which contains only single bonds are said to be
The Alkanes are named following some rules:
- The name of the alkane is given by the number of carbon atoms present in the chain. It is said to be Root of the alkane.
Root = number of carbon atoms in chain.
- To name the root, for one carbon atom, the root name use is meth-. For two carbon atoms, the root name is eth-, for three carbon atoms, it is prop-, for four carbon atoms, it is but-, for five carbon atoms, it is pent- and so on.
- The root name is followed by Suffix. Suffix indicates the
functional group present in the compound. It is placed after the root name.
Suffix = name of the functional group present in the compound.
- The root name also contains Prefix. Prefix is the groups attached to the root. It indicates the branched carbon atoms on the root chain and name according to the root specifying the carbon number on which it is placed. It contains –yl in name end. The prefix is placed before the root name.
Prefix = name of the branched carbon atoms on chain.
- The name of the alkane is given in the form
Prefix + Root + Suffix
(b)
Interpretation:
The systematic name for the below given compound has to be given.
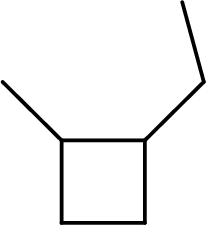
Concept Introduction:
The hydrocarbons which contains only single bonds are said to be Alkanes. When alkane loses two hydrogens and forms a cyclic ring, it is said to be cycloalkane. The general formula for cycloalkanes can be given as
The Alkanes are named following some rules:
- The name of the cycloalkane is given by the number of carbon atoms present in the ring. It is said to be Root of the cycloalkane.
Root = number of carbon atoms in ring.
- To name the root, for one carbon atom, the root name use is meth-. For two carbon atoms, the root name is eth-, for three carbon atoms, it is prop-, for four carbon atoms, it is but-, for five carbon atoms, it is pent- and so on.
- The root name is followed by Suffix. Suffix indicates the functional group present in the compound. It is placed after the root name.
Suffix = name of the functional group present in the compound.
- The root name also contains Prefix. Prefix is the groups attached to the root. It indicates the branched carbon atoms on the root ring and name according to the root specifying the carbon number on which it is placed. It contains –yl in name end. The prefix is placed before the root name.
Prefix = name of the branched carbon atoms on ring.
- The name of the cycloalkane is given in the form
Prefix + Root + Suffix
(c)
Interpretation:
The systematic name for the given compound has to be given and the geometric isomer present in the compound has to be identified.
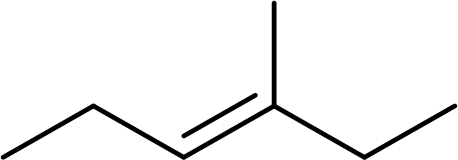
Concept Introduction:
The hydrocarbons which contains double bonds are said to be
The Alkenes are named following some rules:
- The name of the alkene is given by the number of carbon atoms including the double bonded carbon atoms in the chain. It is said to be Root of the alkene.
Root = number of carbon atoms in chain including the double bond.
- To name the root, for one carbon atom, the root name use is meth-. For two carbon atoms, the root name is eth-, for three carbon atoms, it is prop-, for four carbon atoms, it is but-, for five carbon atoms, it is pent- and so on.
- The root name is followed by Suffix. Suffix indicates the functional group present in the compound. It is placed after the root name.
Suffix = name of the functional group present in the compound.
- The root name also contains Prefix. Prefix is the groups attached to the root. It indicates the branched carbon atoms on the root ring and name according to the root specifying the carbon number on which it is placed. It contains –yl in name end. The prefix is placed before the root name.
Prefix = name of the branched carbon atoms on ring.
- The name of the cycloalkane is given in the form
Prefix + Root + Suffix
The geometrical isomers have different orientations of groups around a double bond. The geometric isomers are cis-trans isomers. The isomer which contains same groups or equally prioritized groups on the same side of the double bonded carbon atoms, it is said to be cis-isomer. If the same groups or equally prioritized groups are present on the opposite sides of the double bonded carbon atoms, it is said to be trans-isomer.
(d)
Interpretation:
The systematic name for the given compound has to be given and the chiral centers has to be identified.
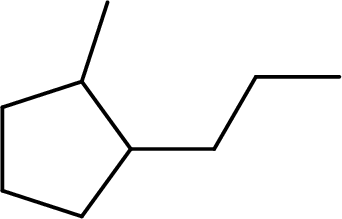
Concept Introduction:
The hydrocarbons which contains only single bonds are said to be Alkanes. When alkane loses two hydrogens and forms a cyclic ring, it is said to be cycloalkane. The general formula for cycloalkanes can be given as
The Alkanes are named following some rules:
- The name of the cycloalkane is given by the number of carbon atoms present in the ring. It is said to be Root of the cycloalkane.
Root = number of carbon atoms in ring.
- To name the root, for one carbon atom, the root name use is meth-. For two carbon atoms, the root name is eth-, for three carbon atoms, it is prop-, for four carbon atoms, it is but-, for five carbon atoms, it is pent- and so on.
- The root name is followed by Suffix. Suffix indicates the functional group present in the compound. It is placed after the root name.
Suffix = name of the functional group present in the compound.
- The root name also contains Prefix. Prefix is the groups attached to the root. It indicates the branched carbon atoms on the root ring and name according to the root specifying the carbon number on which it is placed. It contains –yl in name end. The prefix is placed before the root name.
Prefix = name of the branched carbon atoms on ring.
- The name of the cycloalkane is given in the form
Prefix + Root + Suffix
The atom is said to be as chiral when it is attached to four different groups. It is asymmetrical and does not super-impose on its mirror image. In a compound, the atom which is asymmetric is chiral and is called chiral centre.
Want to see the full answer?
Check out a sample textbook solution
Chapter 15 Solutions
CHEMISTRY >CUSTOM<
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





