
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
12th Edition
ISBN: 9781119664635
Author: Solomons
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 14, Problem 30P
For each of the pairs below, predict specific aspects in their
(a)
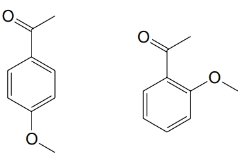
(b)
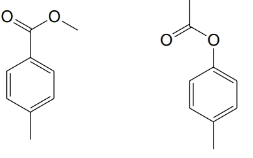
(c)
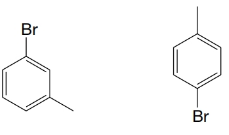
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
(a) The 'H-NMR spectrum of cyclobutanone shows two signals - signal A at 3.00 ppm and signal B at 1.95 ppm. Give the multiplicity of each signal. cyclobutanone
(b) When cyclobutanone is treated with D20 and NaOD, the only signal observable in the 1H-NMR is a singlet at 2.00 ppm. Explain why this is the case. [Note: Deuterium atoms do not display signals in the TH-NMR spectrum]
A graduate student was making some 4-hydroxybutanoic acid and she obtained an excellent yield of a different compound whose 13C NMR is shown here. Propose a structure for this copound.
Treatment of compound C (molecular formula C4H8O) with C6H5MgBr, followed by H2O, affords compound D (molecular formula C10H14O). Compound D has a strong peak in its IR spectrum at 3600–3200 cm−1. The 1H NMR spectral data of C and D are given. What are the structures of C and D?
Compound C signals at 1.3 (singlet, 6 H) and 2.4 (singlet, 2 H) ppm
Compound D signals at 1.2 (singlet, 6 H), 1.6 (singlet, 1 H), 2.7 (singlet, 2 H), and 7.2 (multiplet, 5 H) ppm
Chapter 14 Solutions
ORGANIC CHEMISTRY (LL) W/WILEYPLUS NEXT
Ch. 14 - PRACTICE PROBLEM 14.1 Provide a name for each of...Ch. 14 - Prob. 2PPCh. 14 - Prob. 3PPCh. 14 - Practice Problem 14.4 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.5 Apply the polygon-and-circle...Ch. 14 - Practice Problem 14.6 1,3,5-Cycloheptatriene is...Ch. 14 - Prob. 7PPCh. 14 - Prob. 8PPCh. 14 - Practice Problem 14.9 In 1967 R. Breslow (of...Ch. 14 - Prob. 10PP
Ch. 14 - Practice Problem 14.11 In addition to a signal...Ch. 14 - PRACTICE PROBLEM 14.12
Azulene has an appreciable...Ch. 14 - Practice Problem 14.13 (a) The -Sh group is...Ch. 14 - Practice Problem 14.14
Explain how NMR...Ch. 14 - PRACTICE PROBLEM 14.15 Four benzenoid compounds,...Ch. 14 - Prob. 16PCh. 14 - Write structural formulas and give acceptable...Ch. 14 - Prob. 18PCh. 14 - Prob. 19PCh. 14 - Prob. 20PCh. 14 - Which of the hydrogen atoms shown below is more...Ch. 14 - 14.22 The rings below are joined by a double bond...Ch. 14 - Prob. 23PCh. 14 - 14.24 (a) In 1960 T. Katz (Columbia University)...Ch. 14 - Prob. 25PCh. 14 - Prob. 26PCh. 14 - 14.27 5-Chloro-1,3-cyclopentadiene (below)...Ch. 14 - Prob. 28PCh. 14 - Furan possesses less aromatic character than...Ch. 14 - 14.30 For each of the pairs below, predict...Ch. 14 - Assign structures to each of the compounds A, B,...Ch. 14 - Prob. 32PCh. 14 - Give a structure for compound F that is consistent...Ch. 14 - Prob. 34PCh. 14 - Prob. 35PCh. 14 - The IR and 1H NMR spectra for compound X(C8H10)...Ch. 14 - Prob. 37PCh. 14 - Prob. 38PCh. 14 - 14.39 Given the following information, predict the...Ch. 14 - Consider these reactions: The intermediate A is a...Ch. 14 - Prob. 41PCh. 14 - Compound E has the spectral features given below....Ch. 14 - Draw all of the molecular orbitals for...Ch. 14 - Prob. 1LGPCh. 14 - Prob. 2LGPCh. 14 - 3. The NMR signals for the aromatic hydrogens of...Ch. 14 - Prob. 4LGPCh. 14 - Prob. 5LGP
Additional Science Textbook Solutions
Find more solutions based on key concepts
For Practice 1.1
Is each change physical or chemical? Which kind of property (chemical or physical) is demonst...
Principles of Chemistry: A Molecular Approach (3rd Edition)
What dipeptides would be formed by heating a mixture of valine and N-protected leucine?
Organic Chemistry (8th Edition)
1.3 Obtain a bottle of multivitamins and read the list of ingredients. What are four chemicals from the list?
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
9.1 Calculate the total mass of the reactants and the products for each of the following equations:
Basic Chemistry (5th Edition)
3.1 The reaction between reactant A (blue spheres) and reactant B (red spheres) is shown in the
following diag...
Chemistry: The Central Science (14th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Draw the NMR spectra you would expect for the following compounds.(a) (CH3)2CH¬O¬CH(CH3)2arrow_forwardCompound C has the molecular formula C5H8O. The IR, 1H, 13C, and DEPT NMR spectra of this compound are shown below. (a) Calculate the double bond equivalent of compound C and briefly explain what the values obtains represents. Interpret the IR spectrum. (b) Based on the information provided, determine the structure of compound D.arrow_forwardCompound A with molecular formula C6H10 has two peaks in its 1H NMR spectrum, both of which are singlets (with ratio 9 : 1). Compound A reacts with an acidic aqueous solution containing mercuric sulfate to form compound B, which gives a positive iodoform test and has an 1H NMR spectrum that shows two singlets (with ratio 3 : 1). Identify A and B.arrow_forward
- Compound A has molecular formula C5H10O. It shows three signals in the 1H-NMR spectrum - a doublet of integral 6 at 1.1 ppm, a singlet of integral 3 at 2.14 ppm, and a quintet of integral 1 at 2.58 ppm. Suggest a structure for A and explain your reasoning.arrow_forward(b) One isomer of dimethoxybenzoic acid has 1H NMR spectrum dµ (ppm) 3.85 (6H, s), 6.63 (1H, t, J 2 Hz), and 7.17 (2H, d, J 2Hz), One isomer of coumalic acid has 'H NMR spectrum dн (ppm) 6.41 (1H, d, J 10 Hz), 7.82 (1H, dd, J 2 Hz, 10 Hz) and 8.51 (1H, d, J 2Hz). In each case, which isomer is represented here? The bonds sticking into the centre of the ring can be to any carbon atom. Note: COOH proton is not indicated in the spectra MeO HO₂C CO2H MeO dimethoxybenzoic acid coumalic acidarrow_forwardCompounds A and B are isomers having the molecular formula C4H8O3. Identify A and B on the basis of their 1H NMR spectra.Compound A: δ 1.3 (3H, triplet); 3.6 (2H, quartet); 4.1 (2H, singlet); 11.1 (1H, broad singlet)Compound B: δ 2.6 (2H, triplet); 3.4 (3H, singlet); 3.7 (2H triplet); 11.3 (1H, broad singlet)arrow_forward
- A compound with the molecular formula shown below exhibits a 1H NMR spectrum with only one signal. Deduce the structure in each case. (a) C5H10arrow_forward1 (a) In the following reactions, CI (1) LIAIH, A (2) H20 MCPBA (i) Draw the structure of compounds A and B. (ii) For each reaction, explain the type of reaction involved. (iii) Explain the successful transformation of compound A using mass spectra. (b) Complete the following reactions by filling in missing reactants or product. (i) (1) O3 (2) CH;SCH3 (ii) он (CH3)3(COOH) D Ti(OCH(CH3)2l4 (-)-DETarrow_forwarda) b) Can these three molecules shown below be distinguished by ¹H NMR spectroscopy when the NMR spectrum is recorded in anhydrous (water-free) CDCI3? Give reasons for your answer. NH₂ Can these three molecules shown below be distinguished by ¹H NMR spectroscopy when the NMR spectrum is recorded in deuterated water (D₂O)? Give reasons for your answer. NH₂arrow_forward
- 11. (4) compound X has a molecular formula of C6H8O2, and gave two peaks in its C NMR spectrum but just one in its 1H NMR spectrum (2.5 ppm), IR shows intense absorption peak at 1700 cm provide a structure for compound X.arrow_forwardWhen compound A (C5H12O) is treated with HBr, it forms compound B (C5H11Br). The 1H NMR spectrum of compound A has a 1H singlet, a 3Hdoublet, a 6H doublet, and two 1H multiplets. The 1H NMR spectrum of compound B has a 6H singlet, a 3H triplet, and a 2H quartet. Identifycompounds A and B.arrow_forwardTreatment of compound E (molecular formula C4H8O2) with excess CH3CH2MgBr yields compound F (molecular formula C6H14O) after protonation with H2O. E shows a strong absorption in its IR spectrum at 1743 cm-1. F shows a strong IR absorption at 3600–3200 cm-1. The 1H NMR spectral data of E and F are given. What are the structures of E and F?Compound E signals at 1.2 (triplet, 3 H), 2.0 (singlet, 3 H), and 4.1 (quartet, 2 H) ppmCompound F signals at 0.9 (triplet, 6 H), 1.1 (singlet, 3 H), 1.5 (quartet, 4 H), and 1.55 (singlet, 1 H) ppmarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:9781305446021
Author:Lampman
Publisher:CENGAGE LEARNING - CONSIGNMENT
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY