
MODERN PHYSICS (LOOSELEAF)
4th Edition
ISBN: 9781119495550
Author: Krane
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10, Problem 4Q
How would Figure 10.13 change if the temperature of the gas were increased?
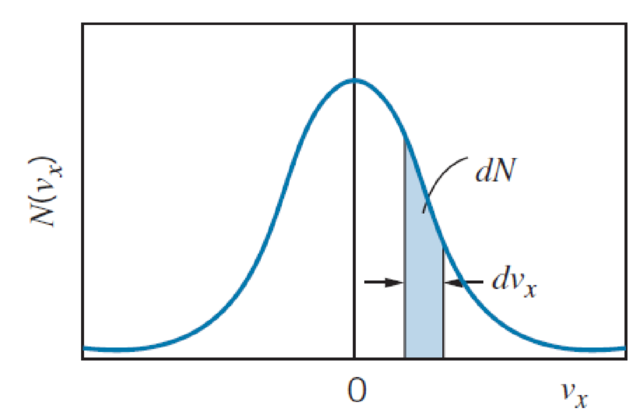
FIGURE 10.13 The Maxwell velocity distribution for gas molecules. The distribution is centered on vx = 0. The shaded strip represents the number of molecules with velocity components between vx and vx + dvx.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Problem 5: Any ideal gas at standard temperature and pressure (STP) has a number density (atoms per unit volume) of p = N/V = 2.68 × 1025 m²3.
How many atoms are there in 11 cubic micrometers, at STP?
N =|
atoms
Eleven molecules have speeds 16, 17, 18, 19,20,21,22,23,24,25, 26 m/s. Calculate the
root-mean-square of this group of molecules. in meters per second. Please give your
answer with one decimal place.
Const
Express your answers using two significant figures separated by comma.
Vz avg, Vy avg = 0,0 m/s
The molecules in a six-particle gas have velocities
v1 = (20i – 30j) m/s
v2 = (50i + 907) m/s
Submit
Previous Answers
v3 = (-90i + 20j) m/s
v4 = 30i m/s
vs = (40i – 40j) m/s
v1 = (-50; – 403) m/s
v Correct
Part B
Calculate vavg
Express your answer to two significant figures and include the appropriate units.
HA
m
Vavg = 0
S
Submit
Previous Answers Request Answer
X Incorrect; Try Again; 5 attempts remaining
Part C
Calculate vrms
Express your answer to two significant figures and include the appropriate units.
Vrms = 69 "
Submit
Previous Answers
Chapter 10 Solutions
MODERN PHYSICS (LOOSELEAF)
Additional Science Textbook Solutions
Find more solutions based on key concepts
The capacitance of plates.
Physics: Principles with Applications
In which extrasolar planet system(s) (A–D) is the planet closest to the star?
Lecture- Tutorials for Introductory Astronomy
An electromagnetic wave has a frequency of 12 MHz. What is its wavelength in vacuum?
University Physics Volume 2
Review Question 23.5 Where should you place an object with respect to a convex lens to have an image at exactly...
College Physics
The real part, imaginary part and the absolute value of cos(ix) .
Mathematical Methods in the Physical Sciences
49. In addition to producing images, ultrasound can be used to heat tissues of the body for therapeutic purpose...
College Physics: A Strategic Approach (3rd Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Consider an ideal gas with an absolute temperature of ?1.T1. To what temperature would the gas need to be heated to double its pressure? Express the answer in terms of ?1.T1. ?2= Consider an ideal gas with a volume of ?1.V1. To what volume would the gas need to be compressed to double its pressure? Express the answer in terms of ?1.V1. ?2=V2=arrow_forwardIn a hypothetical diesel engine the fuel is compressed from 528mL to 39.7 mL. This rasies the temperature of the fuel form 25°C to its ignition temperature of 210°C. If the fuel begins at 1.00atm, what is the pressure of the fuel at its ignition temperature.? Express your answer to 1 decimal place. Be sure to use the proper abbreviation of the UNITSarrow_forwardAn ideal gas is enclosed within a container by a moveable piston. If the final temperature is two times the initial temperature and the volume is reduced to one-fourth of its initial value, what will the final pressure of the gas be relative to its initial pressure, P1?arrow_forward
- Given the ideal gas law P V = k T, where k> 0 is a constant. We have the equation for V in terms of P and T. Finding the rate of change of the volume with respect to temperature at constant pressure, the interpretation of the result is: 1 Because this partial derivative is negative, the volume decreases as the temperature decreases at a fixed pressure. . 2. Because this partial derivative is negative, the volume increases as the temperature increases at a fixed pressure. 3. Because this partial derivative is positive, the volume increases as the temperature decreases at a fixed pressure. 4. Because this partial derivative is positive, the volume increases as the temperature increases at a fixed pressure.arrow_forwardA gas at T0 and atmospheric pressure fills a cylinder. The gas is transferred to a new cylinder with three times the volume, after which the pressure is half the original pressure. What is the new temperature of the gas? Express your answer in terms of T0arrow_forwardA (1.1x10^1) liter bottle is filled with nitrogen (N2) at STP (Standard Temperature and Pressure is 1 atm and 273 K) and closed tight. If the temperature is raised to 100° C, what will be the new pressure in SI units to two significant figures. Note: Your answer is assumed to be reduced to the highest power possible. Your Answer: x10 Answerarrow_forward
- In solid, atoms are spaced closer than in liquid, as shown in Figure 2. Density of a substance is defined as its mass per unit volume. Therefore, density of a solid is typically greater than the density of liquid. However, this is not true for ice (solid water) and liquid water as the density of solid is less that the liquid (Ice floats in water). Explain why this discrepancy occurs in terms of the mass and volume. Solid Liquid Gasarrow_forwardThe Arhennius viscosity model describes how viscosity u depends on temperature 1: u = uo e E/RT 1 DVD DVD Here u is viscosity (Pa.s), I is the temperature (in "Kelvin), E is the activation energy (J mol¹), R is the universal gas constant (R = 8.3145 J mol¹¹ K¹) and U is a constant (Pa s). Ensure all your numerical answers are provided, accurate to 4 significant figures. Linearise this non-linear equation to allow the least squares fitting, i.e. write it in the form y = a + a₁x. Identify the independent (x) and dependent (y) variables and write the linearised equation in the answer boxes, clearly defining what ao and a₁ are equal to in terms of up, E and R. y: ao: a₁: X: IOHO OHO Manarrow_forwardPlease answer all parts: Problem 3: There are lots of examples of ideal gases in the universe, and they exist in many different conditions. In this problem we will examine what the temperature of these various phenomena are. Part (a) Give an expression for the temperature of an ideal gas in terms of pressure P, particle density per unit volume ρ, and fundamental constants. T = ______ Part (b) Near the surface of Venus, its atmosphere has a pressure fv= 91 times the pressure of Earth's atmosphere, and a particle density of around ρv = 0.91 × 1027 m-3. What is the temperature of Venus' atmosphere (in C) near the surface? Part (c) The Orion nebula is one of the brightest diffuse nebulae in the sky (look for it in the winter, just below the three bright stars in Orion's belt). It is a very complicated mess of gas, dust, young star systems, and brown dwarfs, but let's estimate its temperature if we assume it is a uniform ideal gas. Assume it is a sphere of radius r = 5.7 × 1015 m…arrow_forward
- λ=kT/21/2σp is the equation of mean free path. What is the relation of mean free path to temperature, pressure, and molecular cross section. Sketch a plot of the relationship between mean free path and the molecular cross section. Label your axes.arrow_forwardA gas made up of atoms escapes through a pinhole 0.690 times as fast as Ar gas. Write the chemical formula of the gas.arrow_forwardThe accepted value of the density of aluminum at standard temperature and pressure is 2.70 g/cm³ What is the discrepancy between the accepted density and your experimental density in g/cm²? Is this discrepancy significant? QUESTION 9 The accepted value of the density of aluminum is 2.70 g/cm³ while our computed value is 2.8 g/cm³ What is the percent uncertainty of our experimental density (observing correct significant figures)? a. 0.037 Ob.37 c.3.70 d.4 0.5 poinarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON
The Laws of Thermodynamics, Entropy, and Gibbs Free Energy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=8N1BxHgsoOw;License: Standard YouTube License, CC-BY