
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Figure CQ10.14 shows a metal washer being heated by a Bunsen burner. The red arrows in options a, b, and c indicate the possible directions of expansion caused by the heating. Which option correctly illustrates the washer’s expansion?
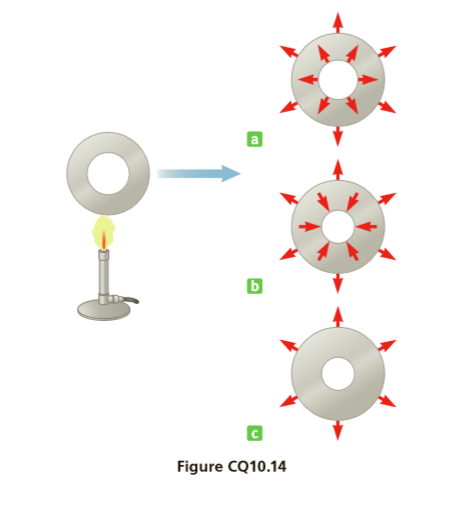
Transcribed Image Text:Figure CQ10.14
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- a 6.0 cm diameter cyliinder of nitrogen gas has a 4.0 cm thick movable copper piston. the cylinder is oriented vertically as shown in the figure, and the air above the piston is evacuated. when the gas temperature is 25 degree C the piston floats 20 cm above the bottom of the cylinder then 1.5 J of heat energy are transferred to the gas. what is the new equilibrium temperature of the gas in degree Carrow_forwardThe phenomena of "wind chill" is a complicated combination of heat transfer through convection and conduction. The following table estimates the effective temperatures due to wind chill for a variety of air temperatures and wind speeds. a. At what temperature, in degrees Celsius, does still air cause the same effective temperature as -5°C air moving at 15 m/s?arrow_forwardQuestion 4. A 5.0 kg block of aluminium is heated from 20°C to 90°C at atmospheric pressure. Find (a) the work done by the aluminium, (b) the amount of energy transferred to it by heat, and (c) the increase in internal energy.arrow_forward
- You have a spherical heater, outside diameter = 3.40 cm, immersed in a container of water. In order to keep the water in the container heated to a constant temperature of 35.0°C you adjust the temperature of the spherical heater. You reach a steady-state condition when the surface temperature of the spherical heater is at 79.0°C. Assuming the electrical efficiency of the heater is 100.0%, calculate the power required by the heater (i.e., calculate q). Ignore radiation.arrow_forwardEstilos Edición P5. A rigid container contains water vapor at 250°C and an unknown pressure. When the container cools to 150°C, the vapor begins to condense. Estimate the initial pressure in the container. Plot the thermodynamic process on a phase diagram. Answer: 600 kPa.arrow_forwardA cylinder container is divided into two equal sections by thermally isolated, frictionless piston,as shown in the figure. One section contains water and the other air. The cylinder is isolated except the one face of the water section. Each section has an initial volume of 100 liter. The initial temperature of the air is 40 degees C and the water is 90 degrees C with steam quality of 10%. The water are heated slowly until the entire section of the water is filled with saturated steam. Find the final pressure and the amount of heat transported to the container. Assume: the heat transfer is a reversible process. Assume for ideal gas the following relation between p and v: (p2/p1)^((k-1)/k) = (v1/v2)^k-1 ; k=1.4arrow_forward
- Aàarrow_forwardIn this problem you will consider the effect that thermal expansion due to temperature will have on Archimedes' principle. Take the densities of water and copper at 0ºC to be 1.00 × 103 kg/m3 and 8.90 × 103 kg/m3, respectively. a. Calculate the fraction of a copper block’s weight that is supported by the buoyant force at 0°C. b. Calculate the fraction of a copper block’s weight that is supported by the buoyant force at 95°C. Assume the volume expansion coefficient of copper is βC = 5.10 × 10-5 1/°C.arrow_forwardD,e,farrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON