
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
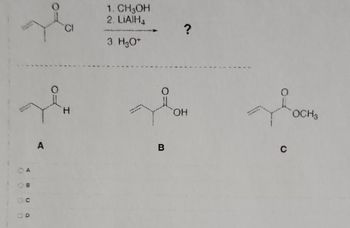
Transcribed Image Text:CD
A
Н
1. CH3OH
2. LiAIH1
3. H2O+
В
?
OH
0
с
OCH 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- CrO3 OH d. H,SO4, H2O е. [1] Mg HO, [2] CO2 [3] H30* f. F Ag20, NH4OH OHarrow_forwardWhich molecule becomes a cation in our body? a b c d earrow_forwardw CHAPTER 4-STOICHIOMETRY: QUANTITATIVE INFORMATION ABOU → Previous Page Page 5 5 of 9 of 9 Next > If you dilute 35.0 mL of 2.25 M hydrochloric acid to 550. mL, what is the molar concentration of the dilute acid? OCT 12 Molar concentration = 2 2 tv # 3 M NIEZA $ W Document3.docx 115 54 FANTASAAYOerene % 5 181WP Conver MacBook Pro 6 NA Oct 10. 2022 at E Awearrow_forward
- Classify the following reactions: a. CH4 + 2 O2 ⟶ CO2 + 2 H2Oi. Combustionii. Double replacementiii. Single replacementiv. Synthesis/combinationv. Decomposition b. N2 + 3 Cl2 ⟶ 2 NCl3i. Combustionii. Double replacementiii. Single replacementiv. Synthesis/combinationv. Decomposition c. 2 Al + 3 CuSO4 ⟶ Al2(SO4)3 + 3 Cui. Combustionii. Double replacementiii. Single replacementiv. Synthesis/combinationv. Decompositionarrow_forwarda laccd sign in - Search ||| tab hift T 0 caps lock % esc O CHEMICAL REACTIONS Percent yield of chemical reactions Be sure your answer has the correct number of significant digits in it. Explanation K- →1 V Liquid octane (CH₂(CH₂) CH₂) reacts with gaseous oxygen gas (0₂) to produce gaseous carbon dioxide (CO₂) and gaseous water (H₂O). If 10.9 g of water is produced from the reaction of 27.41 g of octane and 176.6 g of oxygen gas, calculate the percent yield of water. m X ? https://www-awu.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IvUrTNdLZh5A8CnG03PBGuXr8iCPa7ZMmym Type here to search A 2 Z P Check ALEKS W S # X * 3 X alt E *** f4 $ D 70 4 C X R % 100 Mc Graw He f6 T V McGraw-Hill Education Campus X 4- G (0) 17 B Y ♫+ & 7 H no f8 © 2022 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access a ? KAA * J A ALEKS-Shushanik Babayan - Le X + 96sW-8PcvpF38ZsC... A N fg 3 K f10 0/5 L f12 93°F insert alt Shush 11 prt sc ] bac pause ctriarrow_forwardChapler 6 Tost Redo lI) 6.531 9 sodium hydrox.de soluton are added. to 8.426 g of Tron () chloride solutim. How much iron() hydronde precipitale should be produced? If 4.7329 uas achually produced, what was the % yield ? 2. In the same reachion, it 250 ml o a 03 Somol/L solutin Fe Cls la) was combined with" 5.9639 of sodium hydroride id'a soluhim, ihow much precipitate is formed?arrow_forward
- Consider the given acid ionization constants. Identify the strongest conjugate base. Acid Ka HF(aq) 3.5x10-4 НС-Н: О2 (аq) 6.5х10-5 HC102 (aq) 1.1x10-2 HC2H3O2 (aq) 1.8x10-5 You may want to reference (Page) Section 16.4 while completing this problem.arrow_forwardH perasit 1. EtMgBr A + En ? 2. H₂O 1. H* 2. EtSH ?arrow_forwardMasses : Ca = 40.08 g S= 32.07 g O= 16.00 g A 35.0 mL sample of CaSO4 was evaporated to dryness, leaving 0.967 g of residue. What was the molarity of the original solution? step by step pleasearrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY