
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
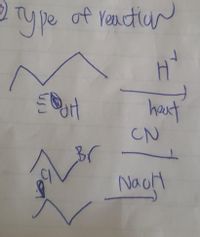
Transcribed Image Text:y pe of Yeuctiun
hast
CN
Nault
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- (64) What is the major organic product for the following sequence?arrow_forward28. 1 || -C-N- The functional group above is referred to as a(n) O amine O amide O acid anhydride Oester 29. The chemical "messengers" between nerve cells that transmit nearrow_forward2. Determine the branch points and reducing ends of amylopectin. A 0.5g sample of amylopectin was analyzed to determine the fraction of the total glucose residues that are branch points in the structure. The sample was methylated and then digested, yielding 50 umol of 2,3-dimethylglucose and 0.4 µmol of 1,2,3,6-tetramethyl glucose. (MWglucose = 162 g/mol, minus H,0) %3D a. What fraction of the total residues are branch points? b. How many reducing ends does the amylopectin have?arrow_forward
- balanced equation for the synthesis of sucrose C12H22O11 + 12O2 → 12CO2 + 11H2O what is the mole ratio of CO2 to H2O H2O to C12H22O11 O2 to CO2 C12 H22O11 to CO2 H2O to O2 O2 to C12H22O11arrow_forward1) Listen triacyl glyceraldhyde results in what chemical reaction (based on prior knowledge)? O hydration O dehydration, intermolecular O dehydration, intramolecular dehydrogenation P Type here to search 421 PM hp ort sc delete 144 end g dn home %23 %24 1% 3. 5 6 8 6. um 4 beckspace Vock ab Q R Y U home D IE G H II J K lock A S enter 4 6. ししに pause V B M t shitt 2 3 end alt ctrl in altarrow_forwardWhat is the integration of stilbenearrow_forward
- ) syatheriae you may ese anly carbaus yeu need to se. Exarple -> you can use a to cueate oner Exerp make Den use it.arrow_forwardThe biruet method uses what reaction to quantify total protein in serum? Question 4 options: A) Cupric ions + peptide bonds in alkaline medium --> violet colored chelate B) Ferric ions + carboxyl bonds in alkaline medium --> aqua colored chelate C) Cupric ions + carboxyl bonds in acidic medium --> aqua clored chelate D) Nitrogen determined via titration with HClarrow_forwardTrial vol. of starch sol'n vol. of vol. of NazS203 diluted vol. of diluted vol. of K2S208 diluted KNO3 conc. of KI conc. of KI conc. of 0.20 M NazS203 0.010 M 0.20 M K2S208 0.10 M 1.0 mL 14.0 mL 5.0 mL 20.0 mL 10.0 mL 1.0 mL 9.0 mL 5.0 mL 20.0 mL 15.0 mL 1.0 mL 4.0 mL 5.0 mL 20.0 mL 20.0 mL 1.0 mL 9.0 mL 5.0 mL 15.0 mL 20.0 mL 1.0 mL 14.0 mL 5.0 mL 10.0 mL 20.0 mL Respond to the following completely in concise full sentences showing any calculations that are needed to 190 32 answer fully. Calculate the concentration of the sodium thiosulfate, Na2S203, the potassium peroxydisulfate, K2S208, and the potassium iodide, KI, found in the reaction mixtures listed in the table above. Remember the total volume in each trial is 50.0 mL. 1. To60x 7032 1L 3,arrow_forward
- (Q16) What is the pH of a solution that is 0.737 M in ethylammonium chloride (CH3CH2NH3CI)? The Kb of ethylammonia (CH3CH2NH2) is 5.6 x 10-4.arrow_forward25-15 Give the general classification of each compound. (a) glyceryl tripalmitate (b) CH3–(CH2)10—CH, 0 S-O- Na+ O sodium lauryl sulfate (in shampoo) CH₂ (d) CH3 (e) H₂C caryophyllene (from cloves) H₂C O (c) CH3–(CH2)3–0–C−(CH2)16–CH3 tetradecyl octadecanoate COOH (f) H H OH PGA₂ H₂C H OH LC=CH H H norethindrone (a synthetic hormone)arrow_forwardDraw the structure of the major organic product(s) of the reaction CI HO You do not have to consider stereochemistry. • All carboxyl and amino groups should be drawn in the neutral form. Draw one structure per sketcher Add additional sketchers using the drop-down menu in the bottom right corner. Separate multiple products using the + sign from the drop-down menu. C. Adoi 000• [Farrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY