
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
Question 1 is just the details and question 2 is what I need help with.
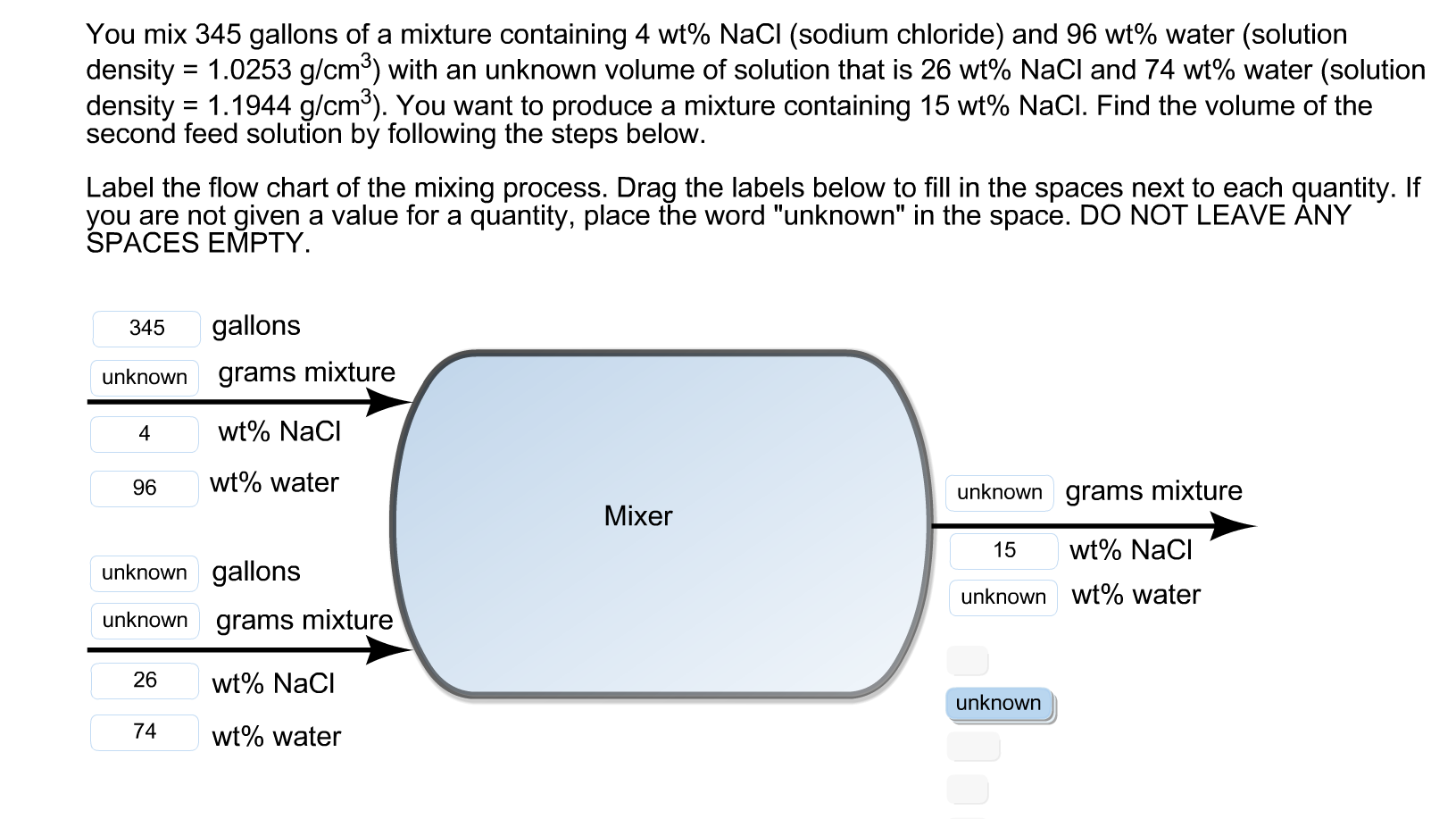
Transcribed Image Text:You mix 345 gallons of a mixture containing 4 wt% NaCl (sodium chloride) and 96 wt% water (solution
density = 1.0253 g/cm³) with an unknown volume of solution that is 26 wt% NaCl and 74 wt% water (solution
density = 1.1944 g/cm³). You want to produce a mixture containing 15 wt% NaCl. Find the volume of the
second feed solution by following the steps below.
Label the flow chart of the mixing process. Drag the labels below to fill in the spaces next to each quantity. If
you are not given a value for a quantity, place the word "unknown" in the space. DO NOT LEAVE ÁNY
SPACES EMPTY.
gallons
345
grams mixture
unknown
wt% NaCl
4
wt% water
96
unknown grams mixture
Mixer
wt% Naci
15
unknown gallons
unknown wt% water
unknown
grams mixture
26
wt% NaCl
unknown
74
wt% water

Transcribed Image Text:Which independent equations can you write to solve for the volume of the second feed solution. Check all
that apply.
Mass balance on water
Volume balance on NaCl
The sum of the weight fractions of the 26 wt% NaCl solution equals 1.
The density of the 4 wt% NaCl solution relates the solution mass to volume.
Volume balance on the total amount of fluid
Volume balance on water
The density of the 26 wt% NaCl solution relates the solution mass to volume.
Mass balance on NaCl
The sum of the weight fractions of the product solution equals 1.
The sum of the weight fractions of the 4 wt% NaCl solution equals 1.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Similar questions
- What are the ideal flow-pattern models?arrow_forwardwrite a note on 2 Dimensional Defects(All of them), Electron Microscope(TEM, SEM, STM) support your writing with picturesarrow_forwardAluminum is not as strong as steel. What other factor needs to be considered when comparing the desirability of aluminum versus steel if strength is an important consideration for a design?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The