
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Pleaase don't provide handwritten solution ....
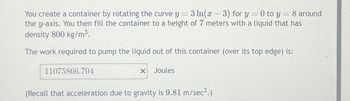
Transcribed Image Text:You create a container by rotating the curve y = 3 ln(x − 3) for y = 0 to y = 8 around
-
the y-axis. You then fill the container to a height of 7 meters with a liquid that has
density 800 kg/m³.
The work required to pump the liquid out of this container (over its top edge) is:
11075866.704
X
Joules
(Recall that acceleration due to gravity is 9.81 m/sec².)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 1 steps with 3 images

Knowledge Booster
Similar questions
- Pls help ASAP. Pls show all work and do all parts of the question.arrow_forwardsin@ =h/l Figure 3 heter to fiqure 3 Guen a track length of 90 cm and a neightd differenie of 18čm,whad 18 Sin e.arrow_forwardMay I ask for a detailed explanation of this figure ( retrieved from Chunhong Long and Jin Yu, “Balancing Non-Equilibrium Driving with Nucleotide Selectivity at Kinetic Checkpoints in Polymerase Fidelity Control”, Entropy 20, 306 (2018), https://pubmed.ncbi.nlm.nih.gov/33265397/ ) ? Description of figure: The kinetic scheme for the three-state nucleotide addition cycle (NAC) with selection. Since the cognate (right) and non-cognate (wrong) nucleotide species are differentiated in the substrate state III, one splits the cycle into two pathways for the right and wrong substrate species. Correspondingly, one has a population vector Π=(PI,PII,PIII^r,PIII^w)^T to describe the overall state probability distributions.arrow_forward
- 1.4 Plot the energy band-gap Eg versus the lattice constant for the two ternary compounds, In¡ „Ga,As and Al,InAs. Label the values for the binary compounds, InAs, GaAs, AIAS, and InP. The band-gap formulas at 300K are E,(In „Ga,As) = (0.36 + 0.505x + 0,555x² Eg(Al,In „As) = 0.36 + 2.35x + 0.24x?. Show on your plot the locations (and values) of the lattice match conditions for the InP substrate.arrow_forward2arrow_forwardNow assume that both the H and the I move when the molecule rotates so the rotating HI moleculeis a rigid rotator and calculate: c) the rotator’s moment of inertiad) the greatest wavelength of radiation that can rotationally excite the molecule.arrow_forward
- The efficiency of an electronic structure computation for a polyatomic molecule depends on both the size of the basis set used and the computational approach utilized, but only one of these factors affects the accuracy of the results. True Falsearrow_forwardWrite the determination of exciton, an excited state formed in an organic semiconductor. Also, what is the diffusion length (such as OPV) of exciton in a general element?arrow_forwardThere are three atoms: A, B, and C. The graphs of potential energy for the pairs AB and BC are shown below (the scale of the axes is the same for both graphs). Which of the following statements is correct? If there is more than one, you must select all of them. r TH U (AB) The AB bond has a smaller magnitude of binding energy than the BC bond. Breaking the AB bond releases more energy than breaking the BC bond. The reaction A + BC -> AB + C is exothermic. U (BC) None of the statements is correct. 1arrow_forward
- ... ... Apps Getting Started Thomson Reuters ADP ezLaborManaarrow_forwardIn this problem you will model the mixing energy of a mixture in a relatively simple way, in order to relate the existence of a solubility gap to molecular behavior. Consider a mixture of A and B molecules that is ideal in every way but one: The potential energy due to the interaction of neighboring molecules depends upon whether the molecules are like or unlike. Let n be the average number of nearest neighbors of any given molecule (perhaps 6 or 8 or 10). Let Uo be the average potential energy associated with the interaction between neighboring molecules that are the same (A-A or B-B), and let UAB be the potential energy associated with the interaction of a neighboring unlike pair (A-B). There are no interactions beyond the range of the nearest neighbors; the values of Uo and UAB are independent of the amounts of A and B; and the entropy of mixing is the same as for an ideal solution. Find a formula for the total potential energy when the system is mixed, in terms of x, the fraction…arrow_forwardPlease don't provide hand writtin solution....arrow_forwardarrow_back_iosSEE MORE QUESTIONSarrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON