
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
These are biochemistry questions:
Questions are part A and B.
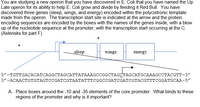
Transcribed Image Text:You are studying a new operon that you have discovered in E. Coli that you have named the Up
Late operon for its ability to help E. Coli grow and divide by feeding it Red Bull. You have
discovered three genes (sleep, wings, and energy) encoded within the polycistronic template
made from the operon. The transcription start site is indicated at the arrow and the protein
encoding sequences are encoded by the boxes with the names of the genes inside, with a blow
up of the nucleotide sequence at the promoter, with the transcription start occurring at the C.
(Asterisks for part F)
sleep
wings
energy
5'-тстTGACАСАТСAGGCTAGCATTATAAAGсCGGCTAGCTAGCATGCAAAGCСТАССТТ-3'
3' -ACAACTGTGTAGTCCGATCGTAATATTTCGGCCGATCGATCGTACGTTTCGGATGCAA-5'
A. Place boxes around the -10 and -35 elements of the core promoter. What binds to these
regions of the promoter and why is it important?

Transcribed Image Text:B. What is the first 6 nucleotides of the mRNA sequence generated at this operon?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Similar questions
- Which of the following statements about pH is (are) true: pH is a measure of free hydrogen ion concentration none of these are true both of these are true An increase in hydrogen ion concentration means a decrease in pH scale unitsarrow_forwardChanges to the sequence of nucleotides in DNA, or changes to chromosomes, are calledarrow_forwardPo ~Av Αν Radioactive Thymine (գլոսյա/siunos) 500 400- 300- 200- 100- Radioactive Uracil (counts/minute) Figure 1- Radioactive Thymine E-621 3¶ = = = = 112 0- 100 80- 60- 40- 20 15 20 Time (minutes) Figure 3-Radioactive Uracil 1 1 0- 14 ted States) IT 1 5 10 5 2 10 25 25 20 15 Time (minutes) 1 30 111111 30 V g DNA in broth 35 2- 1.5- 1- 0.5- 0 3 35 Test broth with antibiotic added Control broth with no antibiotic added 1 V 40 glucose concentration to kill the bacteria. 40 Figure 5-DNA in the Broth 2.5- 5 L AaBbCcDdEe AaBbCcDdEe AaBb AaBbC Normal No Spacing Heading 1 Heading 2 Radioactive Methionine 10 (counts/minute) 30,000 25,000- 20,000- 15,000- Figure 2- Radioactive Methionine 10,000- 5,000- 0++ 500- 400- 300- 200- 100- Figure 4- Glucose Concentration 0 25 1 15 20 Time (minutes) LL MacBook Pro 1 30 5 5 f 5 35 W 20 25 15 10 Time (minutes) 10 40 6 LI 25 15 20 Time (minutes) 30 30 35 35 40 40 Focarrow_forward
- Using my chart can you help me with this question. The tests we used were qualitative; they allowed us to determine if a biological molecule was present or absent. In contrast, what type of information would a quantitative test provide?arrow_forwardStudy the following urine test results and complete Tables 1 and Write “+” signs in the appropriate boxes of Table 2 to indicate different medical problems. Tests A B C D E Leukocyte Nitrite Protein pH Blood Specific Gravity Ketone Glucose Table 2: Medical Problems Indicated by Urinalysis Results Diagnoses A B C D E Urinary Tract Infection Diabetes Kidney Stone Liver Disease Kidney Disease Unclean catcharrow_forwardA glucose monitor is an instrument that diabetics use to check their blood sugar. To use it, you insert a 'test strip' into the monitor and apply blood to it, then the blood is sucked into the instrument and the concentration of the sugars in the blood are measured. Like most instruments, it isn't 100% accurate 100% of the time. The informational pamphlet includued with the test strips say that a sample with a concentration of 7.2 mg/ml will give a reading with a standard deviation of 0.2 mg/ml. This means that when measured, a sample of blood with a 7.2 mg/ml glucose concentration will give a reading between mg/ml % of the time. mg/ml andarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON