
Structural Analysis
6th Edition
ISBN: 9781337630931
Author: KASSIMALI, Aslam.
Publisher: Cengage,
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
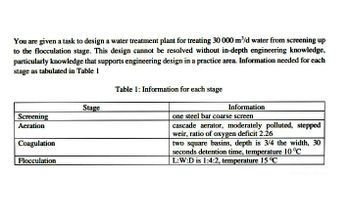
Transcribed Image Text:You are given a task to design a water treatment plant for treating 30 000 m³/d water from screening up
to the flocculation stage. This design cannot be resolved without in-depth engineering knowledge,
particularly knowledge that supports engineering design in a practice area. Information needed for each
stage as tabulated in Table 1
Table 1: Information for each stage
Stage
Information
one steel bar coarse screen
Screening
Aeration
cascade aerator, moderately polluted, stepped
weir, ratio of oxygen deficit 2.26
Coagulation
two square basins, depth is 3/4 the width, 30
seconds detention time, temperature 10 ºC
L:W:D is 1:4:2, temperature 15 °C
Flocculation
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Similar questions
- A groundwater supply has been designated as vulnerable to fecal contamination, and the state has specified a disinfection level of 99.99% (4.0-log) virus inactivation. The peak hourly pumping rate is 2000 gpm from the well field through a 4200-ft pipe with a 16-in diameter to a reservoir in the town. The water temperature is 10°C. A. what is the contact time in the pipeline? B. What chlorine residual is required in the water at the outlet of the pipeline? F3 F4 F5 F6 F7 F8 F9 F10 F11 F12 %23 & 3 4 7 8 T Y D F G K CO LLIarrow_forwardHow do I calculate the required completely mixed aeration tank volume (m3) for the following conditions: Q avg Primary effluent soluble BOD Effluent TSS limit Effluent soluble BOD limit 12,000 m³/d 140 mg/L 20 mg/L 9 mg/L Ks kd μm Y Target MLVSS 67 mg/L 0.14 d-¹ 2.8 d-¹ 0.8 mg VSS/mg BOD 3,000 mg VSS/Larrow_forwardPart darrow_forward
- Hand written and neat and clean answer pleasearrow_forwardPLEASE SHOW CORRECT STEP-BY-STEP SOLUTION. THUMBS UP IF CORRECT. Thank you!arrow_forwardA contaminant is present in an industrial wastewater at a concentration of 268 mg/L and can be degraded according to a first-order reaction. Previous research has identified that K contaminant is regulated at 2.5 mg/L and must be removed prior to discharge to the sewer. 1. 5.2/hr. The a. Identify the required reaction time (in hours) to degrade the contaminant to the target effluent concentration of 2.5 mg/L in an ideal continuously-stirred tank reactor (CSTR). b. Identify the required reaction time (in hours) to degrade the contaminant to the target effluent concentration of 2.5 mg/L in an ideal plug flow reactor (PFR). If the wastewater flowrate is 7,000 gallons/day (gpd), identify the volume required (in gallons and ft') for ideal CSTR and PFR treatment system to achieve the target effluent concentration of 2.5 mg/L. d. Management would like to use two 500 gallon tanks operated as two-CSTRS in series to treat the wastewater at a flowrate of 7,000 (gpd). Will this be satisfactory for…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Structural Analysis (10th Edition)Civil EngineeringISBN:9780134610672Author:Russell C. HibbelerPublisher:PEARSON
Structural Analysis (10th Edition)Civil EngineeringISBN:9780134610672Author:Russell C. HibbelerPublisher:PEARSON Principles of Foundation Engineering (MindTap Cou...Civil EngineeringISBN:9781337705028Author:Braja M. Das, Nagaratnam SivakuganPublisher:Cengage Learning
Principles of Foundation Engineering (MindTap Cou...Civil EngineeringISBN:9781337705028Author:Braja M. Das, Nagaratnam SivakuganPublisher:Cengage Learning Fundamentals of Structural AnalysisCivil EngineeringISBN:9780073398006Author:Kenneth M. Leet Emeritus, Chia-Ming Uang, Joel LanningPublisher:McGraw-Hill Education
Fundamentals of Structural AnalysisCivil EngineeringISBN:9780073398006Author:Kenneth M. Leet Emeritus, Chia-Ming Uang, Joel LanningPublisher:McGraw-Hill Education
 Traffic and Highway EngineeringCivil EngineeringISBN:9781305156241Author:Garber, Nicholas J.Publisher:Cengage Learning
Traffic and Highway EngineeringCivil EngineeringISBN:9781305156241Author:Garber, Nicholas J.Publisher:Cengage Learning


Structural Analysis (10th Edition)
Civil Engineering
ISBN:9780134610672
Author:Russell C. Hibbeler
Publisher:PEARSON

Principles of Foundation Engineering (MindTap Cou...
Civil Engineering
ISBN:9781337705028
Author:Braja M. Das, Nagaratnam Sivakugan
Publisher:Cengage Learning

Fundamentals of Structural Analysis
Civil Engineering
ISBN:9780073398006
Author:Kenneth M. Leet Emeritus, Chia-Ming Uang, Joel Lanning
Publisher:McGraw-Hill Education


Traffic and Highway Engineering
Civil Engineering
ISBN:9781305156241
Author:Garber, Nicholas J.
Publisher:Cengage Learning