Question
Verify the equation of psi and v
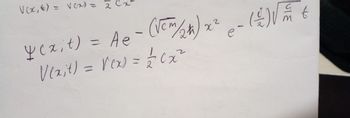
Transcribed Image Text:V(x, €) = vex) = 2
(을) V
y cz₁t) = Ae - (VCM (24) x² – (!) st
2
e
Vexit) = V(x)
= = (x²
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Similar questions
- Find the pressure in psiarrow_forwardThe equation for calculating the work performed when a piston in a cylinder expands against a constant pressure is w = −P ∙ΔV, where ΔV is the change in volume inside the cylinder. How much work is performed (in joules) when a piston pushes against a constant pressure of 1.21 atm, resulting in a volume change of 2.56 mL? (Hint: First determine the units that must be used for each term in the equation.)(101,325 Pa = 1 atm)arrow_forwardIf an ideal gas does not really exist, why do scientists use this this concept?arrow_forward
- An industrial firm supplies compressed air cylinders of volume 0.25 m3 filled to a pressure of 20×106 Pa at 17 ºC. Calculate the number of moles of air in each cylinder.arrow_forwardTwo containers of equal volume each hold samples of the same ideal gas. Container A has 2 times as many molecules as container B. If the gas pressure is the same in the two containers, find the ratio of the the absolute temperatures TA and TB ( i.e TA / TB ) . Calculate to 2 decimals.arrow_forward- What is the density (ing/L) of hydrogen gav at 20.0°C and a provun of 1655 psi?arrow_forward
arrow_back_ios
arrow_forward_ios